During last decades the chemistry of ferrocenes has attracted remarkable attention due to their wide range of interest in material sciences, catalysis and biological assays [1]. Many ferrocenyl compounds display interesting cytotoxic, antitumor, antifungal, antioxidant and DNA-cleaving activity [2].
According the literature a wide variety of heterocyclic scaffolds possess valuable pharmaceutical properties including anticancer effects [1]. Structural motifs containing ferrocene-triazoles and carbohydrate-triazoles are well known for their cytotoxicity and antimicrobial activities. Apart from these activities, the antioxidant behavior of conjugates is also enhanced due to the reversible redox property of ferrocene [3].
We report the simple synthesis of ferrocene-carbohydrate conjugates by click chemistry techniques, which is a method that consists of the regiospecific catalytic cycloaddition of prop-2-ynyloxymethylerrocene and azidosugar leading to the formation of 1,2,3-triazole ring between ferrocene and sugar moiety. The determination of antioxidant activity of the final compounds was made by DPPH method [4].
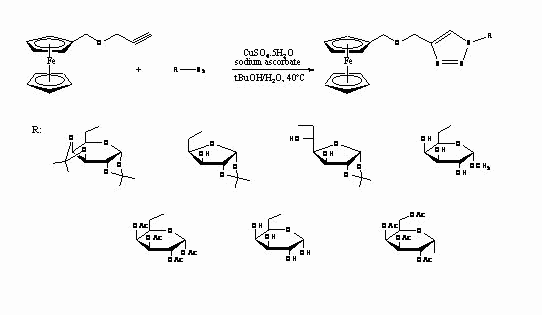
Scheme I - Synthesis of ferrocene-carbohydrates conjugates by click chemistry reaction.References:
1. Á. Gyömöre, A. Csámpai; J. of Organometallic Chem., 2011, 696, 533-539.
2. G. M. Maguene, J. Jakhlal, M. Ladyman, A. Vallin, D. A. Ralambomanana, T. Bousquet, J. Maugein, J. Lebibi, L. Pélinski; Eur. J. of Medicinal Chem., 2011, 46, 31-38.
3. R. Trivedi, S. B. Deepthi, L. Giribabu, B. Sridhar, P. Sujitha, C. G. Kumar, K. V. S. Ramakrishna; Eur. J. Inorg. Chem., 2012, 2267-2277.
4. Kumar, A.; Sharma, P.; Kumari, P.; Lal Kalal, B.; Bioorganic & Medicinal Chemistry Letters, 2011, 21, 4353–4357.
Acknowledgments:
The authors acknowledge FCT for financial support through project PTDC/QUI-QUI/110532/2009.

