Nonsense mutations are a widespread cause for a large number of human genetic diseases, including cystic fibrosis, Duchenne muscular dystrophy, several types of cancer and more. These mutations are characterized by single point alterations in the DNA, where one of the three stop codons (TGA, TAG or TAA) replaces an amino acid-coding codon, leading to a premature termination of the translation and eventually to a nonfunctional protein. Up-to-date there is no effective therapy for these mutations and the only widely used treatment is symptomatic. Aminoglycoside antibiotics were the first small molecule drugs that gave promising results as a potential therapy of such genetic disorders. Gentamicin and geneticin (G418) were recently proven to induce mammalian ribosome suppression of nonsense mutations and partially restore the expression of functional proteins. However, toxicity and relative lack of efficacy at subtoxic doses limit the use of gentamicin for suppression therapy. Although G418 exhibits strongest activity, it is very cytotoxic even at very low doses. Clearly, the challenges in this emerging field should be at deciphering the intimate structural and functional determinants of aminoglycosides as readthrough and toxicity inducers, in order to develop new structures with improved suppression activity and reduced toxicity.
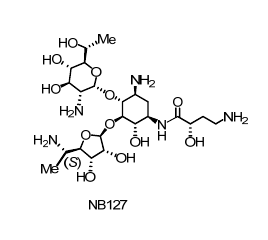
Towards these ends, by using systematic structure-activity-toxicity relationship study we were able to develop a new generation of aminoglycoside, NB127, which lacks antibacterial activity and exhibits similar in vitro and ex vivo activity to that of G418. Furthermore, its cell toxicity is significantly lower than those of gentamicin and G418. Using a series of biochemical assays, we provide proof of principle that antibacterial activity and toxicity of aminoglycosides can be dissected from their suppression activity.