Brown seaweeds polysaccharides fucoidans exhibit anticoagulant, anti-inflammatory, antithrombotic, anticancer and some others biological activities. Their effect depends on fucoidan structure (types of glycosidic bonds, degree and pattern of sulfation, presence of branches) [1,2]. To understand the influence of fucoidan structure on their biological action, we perform the synthesis of oligosaccharides related to fucoidans from different seaweeds. Here we report on the synthesis of disaccharides 6 and 7 related to fucoidan from C. flagelliformis, bearing a-L-fucofuranose residue [3].
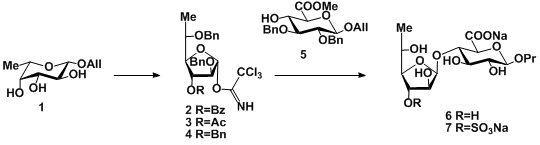
Appropriate protection of β-allyl fucopyranoside 1, further pyranoside-to-furanoside rearrangement under acid promoted per-O-sulfation conditions [4] followed by transformation into trichloroacetimidates gave a series of fucofuranosyl donors 2-4. Their coupling with acceptor 5 gave a-linked disaccharide exclusively in the cases of 3-O-acylated donors 3 and 4, but 1:2 mixture of α- and β-isomers in the case of per-benzylated donor 5, demonstrating strong stereo-controlling effect of 3-O-acyl group in fucofuranosyl donors. This fact could be explained by the anchimeric assistance of acyl group favoring α-fucosylation. Removing of protective groups and selective 3’-O-sulfation gave the target disaccharides 6 and 7.
1. A. Cumashi, N.A. Ushakova, M.E. Preobrazhenskaya, A. D'Incecco, A. Piccoli, L. Totani, N. Tinari, G.E. Morozevich, A.E. Berman, M. A. Bilan, A.I. Usov, N.E. Ustuzhanina, C.J. Sanderson, M. Kelly, G.A. Rabinovich, S. Iacobelli, N.E. Nifantiev, Glycobiology, 2007, 17(5), 541
2. D. Croci, A. Cumashi, N.A. Ushakova, M.E. Preobrazhenskaya, A. Piccoli, L. Totani, N.E. Ustyuzhanina, M.I. Bilan, A.I. Usov, A.A. Grachev, G.E. Morozevich, A.E. Berman, C.J. Sanderson, M.Kelly, P. Di Gregori, C. Rossi, N. Tinari, S. Iacobelli, G. A. Rabinovich, N.E. Nifantiev, PlosOne, 2011, 10.1371
3. M.I. Bilan, E.V. Vinogradova, E.A. Tsvetkova, A.A. Grachev, A.S. Shashkov, N.E. Nifantiev, A.I. Usov. Carbohydr. Res., 2008, 343, 2605
4. V.B. Krylov, Z.M. Kaskova, D.Z. Vinnitskiy, N.E. Ustyuzhanina, A.A. Grachev, A.O. Chizhov, N.E. Nifantiev. Carbohydr. Res. 2011, 346, 540

