Asparagine-linked
oligosaccharides (N-glycans) on
glycoproteins have high diversity and complexity and are involved in a variety
of important physiological events, such as protein quality control, cell-cell
recognition, adhesion, and signal transduction. We have investigated the new
synthetic strategy of N-glycan
library for the bio-functional analysis. The strategy, which could directly
give the glycans linked with the asparagine residue at the reducing-end, makes
it easy to introduce synthetic N-glycans
to glycoproteins or other bioconjugate molecules.
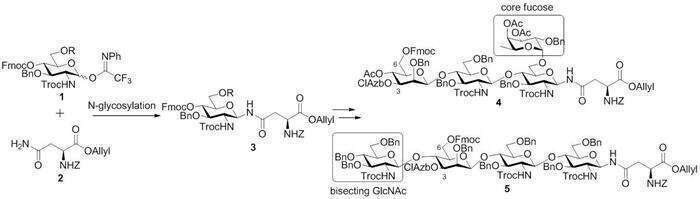
Glycosyl-Asn structure (GlcN-Asn,
3) was first synthesized by N-glycosylation under microfluidic condition, and then oligosaccharide chains were
elongated step by step to give the tetrasaccharides 4 and 5, which contain
core fucose and bisecting GlcNAc structure, respectively (Figure 1). Compounds 4 and 5 are important intermediates for N-glycan library, as selective deprotection of
4-azido-3-chlorobenzyl (ClAzb) group and Fmoc group at 3 and 6 position of
mannose followed by further installation of oligosaccharide units will afford
various N-glycans. Next, sequential
deprotection of 4 and 5 successfully gave the deprotected
oligoglycosyl asparagines. The basic synthetic strategy of N-glycan library was hence established. Efficient a-sialylation under microfluidic
condition and further elongation of sugar chain will also be reported