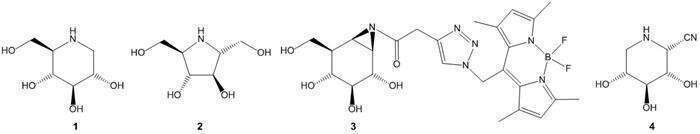
Iminosugars, carbohydrate analogues
with a basic nitrogen instead of oxygen in the endocyclic position such as
compounds 1 and 2, have gained undisputed importance due to their mode of action of
interacting with glycosyl hydrolase as diagnostics and therapeutics in the
context of, for example, Diabetes Typ II, cancer, certain bacterial and viral infections
or lysosomal storage diseases. Furthermore, these compounds have been found to
be very useful tools in the elucidation of the mechanism and structure determination
of the same enzyme class. [1]
Recently, carbohydrate analogues
conjugated to reporter groups such as fluorescent dyes or biotin, for example
compound 3, have been introduced as
attractive probes for activity based protein profiling (ABPP) of glycosyl
hydrolases.[2] We became interested whether iminosugars, when appropriately
equipped with reporter groups, could also be suitable as probes for ABPP. In
this context, cyano-iminosugar derivatives, such as compound 4, can serve as versatile building
blocks for the synthesis of such probes. The cyano group introduces an
additional orthogonal “point” for modification reactions such as the introduction
of reporter groups to the iminosugar scaffold.
A short and efficient preparative
method for the synthesis of differently configured cyano-iminosugar building
blocks as well as follow up chemistry concerning the conjugation of fluorescent
dyes towards the construction of “glycoprobes” will be presented.
[1] A.E.
Stütz, T.M. Wrodnigg, Adv. Carbohydr.
Chem. Biochem., 2011, 66, 187-298. R.J. Nash, A. Kato, C-Y.
Yu, G.W.J. Fleet, Future Med. Chem., 2011,
3, 1513-1521. T.M. Wrodnigg,
A.E.Stütz, Curr. Enz. Inhibit., 2012, 8(1), 47-99.
[2] W.W.
Kallemeijn, K-Y. Li, M.D. Witte, A.R.A. Marques, J. Aten, S. Scheij, J. Jiang,
L.I. Willems, T.M. Voorn-Brouwer, C.P.A.A. van Roomen, R. Ottenhoff, R.G. Boot,
H. van den Elst, M.T.C. Walvoort, B.I. Florea, J.D.C. Codée, G.A. van der Marel,
J.M.F.G. Aerts, H.S. Overkleeftet, Angew.
Chem. Int. Ed., 2012, 51, 12529-12533.

