Lipopolysaccharide (LPS) constitutes the outer leaflet of outer cell membranes of Gram-negative bacteria playing a vital role as an antigenic motif in pathogens. These immunogenic structures are frequently recognized by C-type lectins of the innate immune system1. Previously, ELISA binding studies revealed an untypically high binding affinity of human MBL-A (mannose binding lectin) to isolated oligosaccharides of Acinetobacter LPS2. While the species-specific carbohydrate scaffold has been elucidated3, identification of the carbohydrate-subunit responsible for this potent adhesion has not been accomplished. For a detailed investigation of the binding epitope the synthesis of the truncated core saccharides is necessary. The synthesis of the naïve disaccharides αGlcp-(1g5)-αKdo (Kdo = 3-deoxy-D-manno-oct-2-ulosonic acid) 1a and its phosphorylated pendant αGlcp6P-(1g5)-αKdo 1b has been achieved in good anomeric selectivity and high overall yields. Furthermore, the corresponding non-natural disaccharides αGlcp(6P)-(1g5)-αKdh (Kdh = 3-deoxy-D-lyxo-hept-2-ulosonic acid) 2a and 2b accessible via Malaprade reaction and the setup of the first trisaccharides containing the additional αKdo-(2g4)-αKdo linkage will be presented. Biological studies will lead to a better understanding of the efficient binding interactions of Acinetobacter LPS on a molecular range being valuable knowledge for future medical treatment of antibiotic-resistant pathogens.
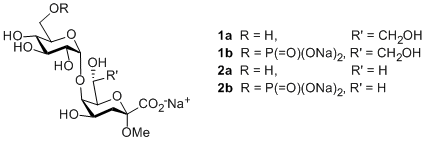
References: 1 Weis, W.I., et al., Immunol. Rev. 1998, 163, 19 – 34; 2 Brade, L., et al., Infect. Immun. 1985, 50, 687 – 694; 3 Vinogradov, E.V. et al., Eur. J. Biochem. 1997, 247, 82 – 90
Aknowledgement: Financial support by the FWF (project: P 24921) is gratefully aknowledged.

