During the last decade, Sharpless concept of click chemistry was transplanted to carbohydrate chemistry1-3. While, the original alkyne-azide concept of click-chemistry is well known, the alternative thiol-click is relatively unexplored. Recently, we have developed one-pot, Thiol Enone Michael Addition (TEMA) click procedure protocol for the synthesis of new family of (1-5)-C-thiodisaccharides4. In continuation of this strategy, we also developed new approach of base catalyzed opening of functionalized epoxides 2, 5-6, and 7 conveniently prepared in our laboratory.
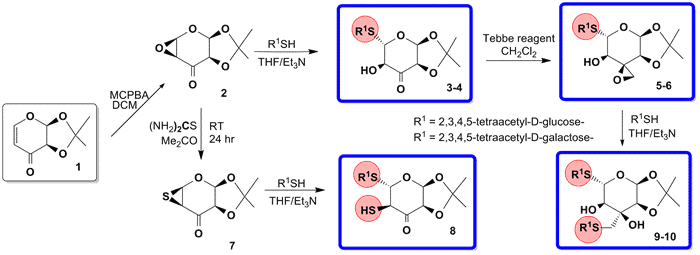
Epoxides 2, 7, undergoes stereoselective base catalyzed opening with D-glucose, and D-galactose 1-thiols. The new functionalized 1-5-S-thiodisccharides 3-4 were subsequently converted into epoxides 5-6 in the reaction with Tebbe reagent and again undergoes base catalyzed opening with D-glucose, and D-galactose 1-thiols via one step thiol-click approach. The exclusive regio- and stereochemistry of epoxide openings proceeds with the formation of adducts 3-4 and 9-10 in high yields. The epoxide 2 was smoothly converted into episulfide 7, which under highly stereoselective base catalyzed opening produced thiol 8 in quantitative yield. New thio-S-trisaccharides 9-10 will be used as new tools for glycobiology and specifically as new inhibitors of galectin-3.5 The preliminary inhibition data of these novel thio-sugars will be discussed in details.
1. Click Chemistry in Glycoscience: New Developments and Strategies. J. Wiley & Sons, NY, 2013. Z. J. Witczak, R. Bielski D, Editors
2. M. Fiore, A. Mara, and A. Dondoni, J. Org. Chem., 2009, 74, 4422-4425.
3. S. G. Gouin, L. Bultel, C. Falentin and J. Kovensky, Eur. J. Org. Chem. 2007, 1160-1162.
4. Z. J. Witczak, D. Lorchak and N. Nguyen, Carbohydr. Res., 2007, 342, 1929-1931.
5. Galectins, J. Wiley & Sons, NY, 2008. A. Klyosov, Z. J. Witczak, D. Platt, Editors.

