D-Fructose is the second most abundant simple sugar in nature, but the chemical synthesis and pharmaceutical research of fructosides are less developed. In recent years, fructopyranosides proved their pharmaceutical value.
We developed a mild and general method for fructopyranosides preparation with N-phenyltrifluoroacetimidate donor, which provided good yield and b-selectivity. The anomeric configuration determination methods and the b-selective fructosylation mechanism were discussed. 2-O-Acetyl-b-D-Fructopyranosides were also found to be good donors for fructopyranoside synthesis. Though simple as Acetyl Fructopyranosides seems, the glycosylation provided interesting results. In some cases, a-D-Fructopyranosides, which were rarely reported, were prepared.
With these two glycosylation methods,several bioactive b-D-frutopyranosides were synthesized.
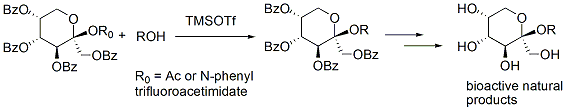
Achyranthes bidentata polysaccharides exhibit interesting pharmacological activity, their pyranosyl congeners were designed and synthesized, which is the first reported work of fructopyranosyl penta- and hexa-saccharides synthesis. These oligomers showed good antitumor activities.
References:
1. Lin, F.; Lian, G.-Y.; Xu, Q.-L.; Zhu, X.-F.Tetrahedron,2013,69, 1128

