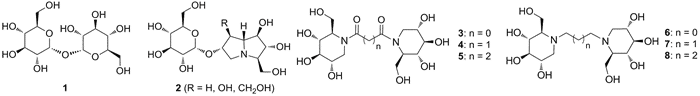
Trehalose (1) is a nonreducing disaccharide in which the two glucose units are linked in an a,a-1,1-glycosidic linkage. This sugar is present in a wide variety of organisms where it may serve as a source of energy and carbon. Trehalose analogs can find different biological applications as: selective probes of Mycobacterium tuberculosis1, antitumor and anti-metastasis agents2, trehalose processing enzymes inhibitors, such as trehalase3 and mycobacterial sulfotransferase4. We recently synthesized some of the most powerful inhibitors of trehalase identified to date, with an imino sugar linked to the sugar moiety in a pseudo disaccharide structure (compounds 2)3. In the present work we wish to report the synthesis of new nojirimycin- based trehalose mimetics (compounds 3-8). Preliminary evaluation of the inhibitory activity against porcine and insect trehalases was performed.
References
1 Backus K. M., Boshoff H. I., Barry C. S., Boutureira O., Patel M. K., D’Hooge F., Lee S. S., Via L. E., Tahlan K., Barry III C. E., Davis B. G. (2011) Nat. Chem. Biol., 7, 228-235.
2 Jiang Y. -L., Tang L. -Q., Miyanaga S., Igarashi Y., Saiki I., Liu Z. -P. (2011) Bioorg. Med. Chem. Lett., 21, 1089–1091.
3 Bini D., Forcella M., Cipolla L., Fusi P., Matassini C., Cardona F. (2011) Eur. J. Org. Chem. DOI: 10.1002/ejoc.201100484 and refs cited therein.
4 Lin F. L., van Halbeek H., Bertozzi C. R. (2007) Carbohyd. Res., 342, 2014–2030.

