The L-hexoses play important roles in Nature and numerous biologically important compounds contain L-hexoses in their structure. For examples, L-gulopyranosides are the key constituents of the antitumor drug Bleomycin A2 and L-iduronic acid is a component of mammalian dermatan sulphate, heparan sulphate, and heparin. L-Mannose is found in some bacteria cell-wall polysaccharides whereas L-galactose has been found in the marine octocoral and is a constituent in some plant biopolymer. Easy excess to L-sugars is therefore highly desirable.
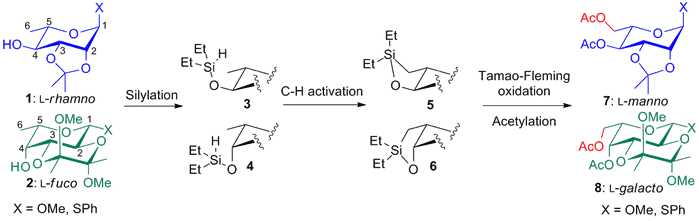
To achieve this, a procedure for the C(sp3)-H activation of 6-deoxy sugar was developed starting from 1 and 2. This protocol is based on a methodology for hydroxylation of methyl groups recently developed by the Hartwig group. Silylation of 4-OH (of 1 or 2) form the (hydrido)silyl 3 & 4 via a hydrogenative silylation. Then iridium-catalyzed C-H activation results in a five-membered oxasilolane (5 & 6), which is oxidized via a Tamao-Fleming oxidation and acetylation to give the L-sugars 7 & 8. The procedure can be performed on methyl pyranosides (X=OMe) and phenylthio pyranosides (X=SPh) in a one-pot (one-purification) procedure in high yields (55-82% over four steps). Applying this procedure synthesis of all 8 L-hexoses is presented.

