Lipopolysaccharide (LPS) is a potent inducer of innate immune response triggered upon binding of the Lipid A portion of LPS by Toll-like receptor 4 (TLR4) – myeloid differentiation factor 2 (MD-2) complex. Disorders in the regulation of TLR4-mediated innate immune signaling can lead to over-production of inflammatory mediators which contributes to the pathogenesis of autoimmune, chronic inflammatory and infectious diseases including arthritis, asthma, sepsis syndrom and septic shock (30% mortality rate), the effective treatment for which is still not available. Based on the comparison of the crystal structures of MD-2/TLR4 complex with bound Lipid A ligands and x-ray structures of a,b-, b,b- and a,a- trehaloses, a series of conformationally constrained Lipid A mimetics based on 1,1'-disaccharide scaffolds have been rationally designed and synthesised. Varying anomeric configuration of the non-reducing disaccharide scaffold and the sites of attachment of functional groups (phosphate and long-chain acyl residues) allowed for rational design and synthesis of TLR4/MD-2 - specific ligands having anti-endotoxic or agonistic properties as antisepsis drug candidates or immunomodulatory agents, respectively.
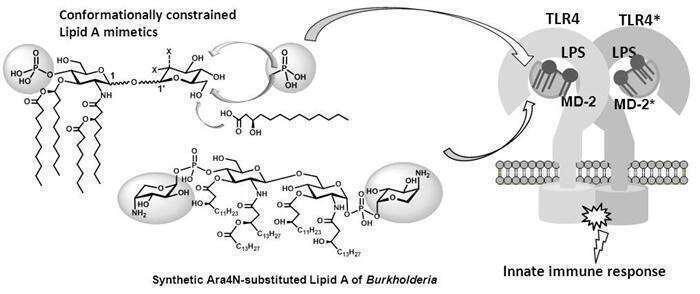
Covalent substitution of Lipid A phosphates by cationic b-L-Ara4N, which is a characteristic feature of Burkholderia LPS, is associated with increased virulence and modulation of TLR4 signaling.2 Since homogeneous preparations of Ara4N-modified B. cepacia Lipid A are not available, it was chemically synthesised to further explore the structure-function relationship in the Lipid A-MD-2/TLR4 complex. Synthesis of amphoteric Lipid A of B. cepacia implied stereoselective introduction of intrinsically labile phosphodiester linkage connecting two anomeric centres.
Acknowledgments: Financial support from Austrian Science Foundation (FWF, P-21276, P-22116) is gratefully acknowledged.
1.
Park, B.S., Song, D.H., Kim, H.M., Choi, B.-S., Lee, H., Lee, J.-O. Nature 2009, 458, 1191
2. Silipo, A., Molinaro, A. et al, Eur. J. Org.Chem. 2006, 4874.

