Burkholderia cepacia complex (Bcc) is a group of Gram-negative bacteria which can cause various lung infections in patients with cystic fibrosis often resulting in a higher mortality of these patients. In order to understand the mechanism of bacterial infection and host recognition, several working groups have studied the structure of the LPS from different types of Bcc in the last years [1, 2].
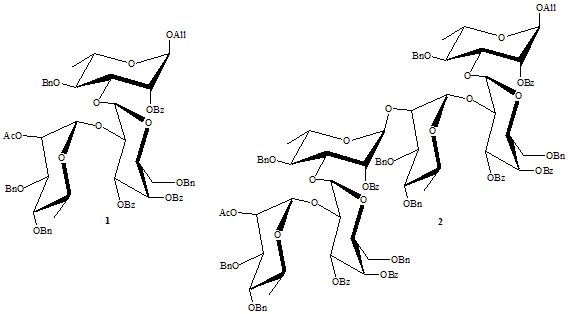
Molinaro et al. have defined the structure of the O-specific polysaccharide from the LPS of Burkholderia anthina [3] which consists of one D-galactose and two L-rhamnose units, linked by 1®2 and 1®3 a-glycosidic bonds, respectively. In order to get a building block for the preparation of higher oligomers, trisaccharide 1 was successfully synthesized and used to prepare hexasaccharide 2. Removal of the allyl group of the trisaccharide offers the preparation of a glycosyl donor whereas the cleavage of the acetyl group leads to a glycosyl acceptor. After deprotection, the trisaccharide and hexasaccharide were used by the group of Molinaro for binding studies with an antibody of Bcc. The total pathway and NMR investigation will be discussed.
1. A. Vinion-Dubiel, J. B. Goldberg, J. Endotoxin Res.9, 201-213, 2003.
2. A. De Soyza, A. Silipo, R. Lanzetta, J. R. Govan, A. Molinaro, Innate Immun.14, 127-144, 2008.
3. S. Carillo, A. Silipo, V. Perino, R. Lanzetta, M. Parrilli, A. Molinaro, Carbohydr. Res.344, 1697-1700, 2009.

