Many
biologically active natural products contain sugar moieties and often the
presence of the glycan is critical for the pharmacology and bioactivity of the
molecule.1 Deoxysugars are an
important class of carbohydrates found in many glycoconjugate type drugs e.g.
Erythromycin, Aclarubicin. Therefore,
the development of a synthetic method to control the stereoselectivity of
glycosylation reactions affording 2-deoxyglycosides is desired.
Herein
we report a mild organocatalytic method for the synthesis of 2-deoxygalactosides,
with excellent yields and α-selectivity.
This method utilises thiourea 1,
which catalyses the addition of an
alcohol 3 to the double bond of the
galactal 2 to form
2-deoxygalactosides 4.2
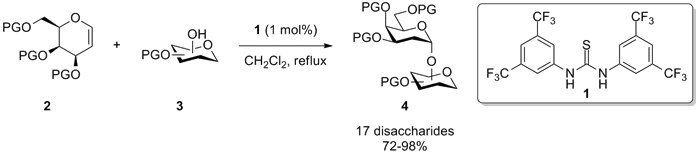
The
method is tolerant of a wide range of protecting groups on both the alcohol and
galactal substrates and is semi-orthogonal to thioglycosylation type reactions,
which allows for one pot tandem chemo-selective glycosylation reactions to afford
trisaccharides.2 The position and stereochemistry of the alcohol.
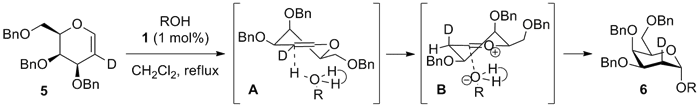
The
high α-selectivity is independent of the acceptor substitution pattern and
reactivity profile.2 Reaction of deuterated galactal 5 showed that the newly formed bonds
are cis to each other. The proposed mechanism is that the initial
thiourea-alcohol complex A delivers
the proton to the least hindered face of the galactal, followed by rapid
collapse of the ion pair intermediate B
to give 6.2
1. a) M. Sastry, D. J. Patel, Biochemistry 1993, 32, 6588–6604;
b) P. T. Daniel, U. Koert, J. Schuppan, Angew. Chem. 2006, 118, 886–908; Angew.
Chem. Int. Ed. 2006, 45, 872–893
2. E. I. Balmond, D. M. Coe, M. C. Galan, E. M. McGarrigle,
Angew. Chem. Int. Ed. 2012, 51, 9152–9155

