Protein glycosylation is a post-translational modification that alters both the structure and the function of many proteins. Several clinically useful cancer biomarkers are in fact glycoproteins (e.g. CEA, HER2). We profiled the serum glycomics of breast cancer samples in two different cohorts. We developed and employed statistical tools to uncover novel glycosylation related biomarkers in the investigated cohorts. In the MicMa cohort of breast cancer samples we are able to identify glycans that are linked to survival.
Two cohorts of breast cancer samples were profiled using (HILIC) technology to obtain the abundance levels of 46 glycan groups. The MDG cohort consists of 169 breast tissue samples, 62 healthy and 107 primary cancer samples. The MicMa cohort consists of 100 primary breast cancer samples and includes rich clinical information as well as extensive genomics information, including mRNA and miRNA profiling in the primary tumor samples.
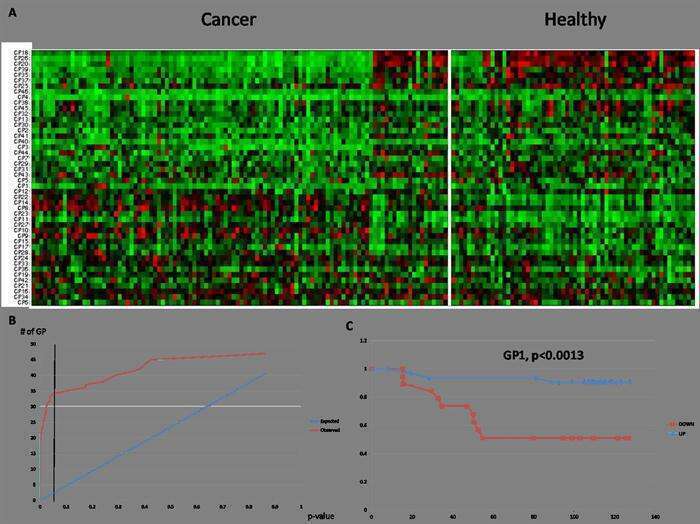
In the MDG cohort we found statistically significant differential abundance of glycans when comparing serum from cancer patients to that coming from control subjects (Fig1, A+B). Specifically, we show that GP18, a fucosylated and syalilated bi-antennary glycan with a bisecting Glc-NAC, is highly abundant in serum from cancer patients and that GP22, which consists of similar structures with no bisection, is under abundant in serum from cancer patients (p
For several GPs a significant association with biological modules is observed. Specifically, we show that GP34, a group rich with syalilated glycans, is significantly associated with genes related to cell migration (p

