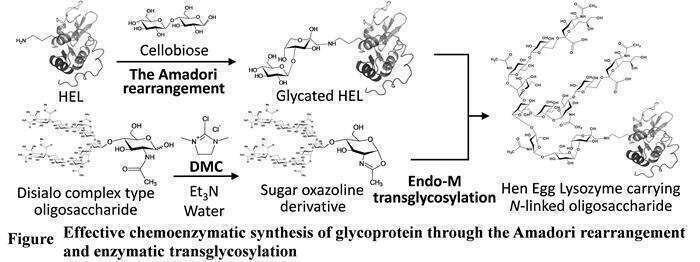
Regioselective introduction of an oligosaccharide into proteins is an attractive task for the improvement of the functional properties of proteins. The Amadori rearrangement is known to be a spontaneous reaction between amino group of protein and reducing sugar and occurs in aqueous solution after the formation of Schiff base. Successive glycosidase-catalyzed transglycosylation onto the resulting "glycated" protein becomes one of the promising tools for the preparation of neo-glycoproteins. Recently, two effective efforts for the formation of neoglycoproteins have been reported: direct transformation of sugar oxazoline derivatives of oligosaccharides (Oligo-oxa) by using 2-chloro-1,3-dimethylimidazolium chloride (DMC)[1] and development of glycosynthase-like mutant of M. hiemalis endoglycosidase (Endo-M N175Q).[2] In this paper, we describe an improved process by combination use of direct transformation of Oligo-oxa and Endo-M N175Q catalyzed transglycosylation reaction to glycated protein (Figure). As a model protein, we employed hen egg lysozyme (HEL) in this study. Endo-M N175Q and sugar oxazoline derivative of disialo-complex-type-oligosaccharide were added into the reaction mixture after the glycation reaction of the HEL. When the reaction mixture was measured by MALDI-TOF-MS, magnified intensity of signals corresponding to HEL having an N-linked oligosaccharide was observed, reaction products revealed that a novel HEL having an oligosaccharide was formed. Based on the fact that intensity of signals corresponding to HEL having an N-linked oligosaccharide was stronger than previous result, this improved process is effective for the formation of neoglycoprotein through the Amadori rearrangement.
[1] M. Noguchi, et al., J. Org. Chem. 2009, 74, 2210.
[2] M. Umekawa et al., Biochim Biophys Acta Gen Subj. 2010, 1800, 1203.

