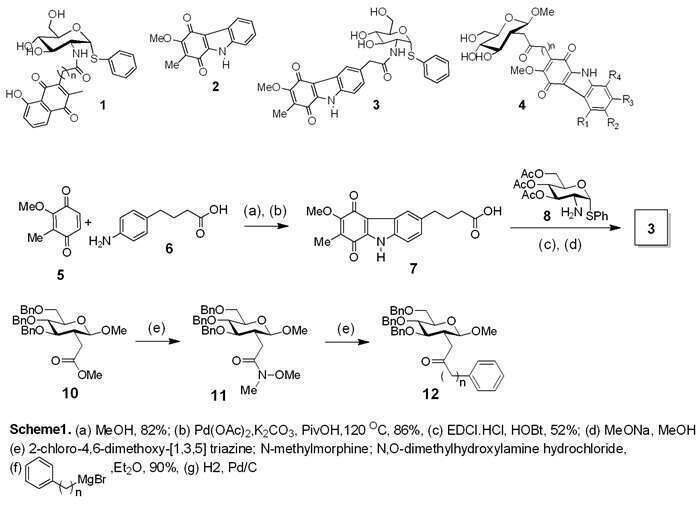
Mycothiol is found only in Actinobacteria1,2 including M. tuberculosis, and appears to play an important role in the bacterium’s defence against xenobiotics and oxidative stress. The biosynthetic pathway to this pseudo-disaccharide has been elucidated and a range of mycothiol-dependent enzymes have been identified. Recent work suggests that compounds 1, which have naphthoquinonyl units tethered to the pseudodisaccharide core, have significant inhibitory activity against mshB and mca in the biosynthetic pathway.3,4 The objective in this work was to build on these findings, together with the independent discovery of anti-TB activity associated with carbazole quinones5,6 such as 2, to construct a new class of hybrid molecules 3 which have the carbazole quinone motif tethered to the pseudodisaccharide core. In addition, a synthetic route was sought to isosteres 4 of mycothiol in which the amide NH is replaced by a methylene group.
The amide-linked hybrid molecule 3 was assembled as shown in Scheme 1, using the Knölker strategy6 of Pd-mediated coupling of quinone 5 to aromatic amine 6, followed by coupling of acid 7 with phenyl-2-amino-1-thioglucoside 8. Methodology has been established for coupling the carbazole and glucose units via a carbon tether, involving reactions of Weinreb amide 11 with Grignard reagents to give 2-C-alkyl derivatives 12.
References:
1. V. K. Jothivasan and C. J. Hamilton, Nat. Prod. Rep., 2008, 25, 1091.
2. G. L. Newton, N. Buchmeier and R. C. Fahey, Microbiology and Molecular Biology Reviews, 2008, 72, 471.
3. D.W. Gammon, R. Hunter, D.J. Steenkamp and T.T. Mudzunga, Bioorg. Med. Chem. Lett. 2003, 13, 2045-9.
4. R. Slättegård, D. W. Gammon and S. Oscarson, Carbohydr. Res. 2007, 342, 1943-1946.
5. I.L. Rogers, D.W. Gammon and K. J. Naidoo, Carbohydr. Res. 2013, doi:http://dx.doi.org/10.1016/j.carres.2013.02.001
6. H-J Knölker, Current Organic Synthesis, 2004, 1, 309.

