Mycoplasmas, members of the class Mollicutes, are the smallest self-replicating prokaryotes. The lack of cell wall in these organisms makes plasma membrane regulation essential for their survival.1 Glycoglycerolipids are key structural components of the plasma membrane involved in bilayer properties and stability.2 This type of lipids is not found in animal cell membranes. Therefore it is proposed that glycoglycerolipid synthases are potential specific targets for drug discovery against mycoplasma infections.
A membrane-associated glycosyltransferase, GT MG517, has been identified in Mycoplasma genitalium, which sequentially produces monoglycosyl- and diglycosyldiacylglycerols (MGDAG and DGDAG), two key components of mycoplasma plasma membrane.3
Given the difficulties to obtain the MG517 crystal structure, our group has proposed 4 bioinformatic models for MG517 structure. Based on those models, key residues interacting with the UDP-glucose donor in the active site have been pointed out. In this work we analyze individually the effect of point mutations on those residues, aiming to validate the proposed models and to obtain a better understanding of their functional role in MG517. We have identified an essential position for catalysis (E193), proposed to be the catalytic base and an interesting position (Y169) that allowed a nearly four times activity enhancement when mutated into phenylalanine.
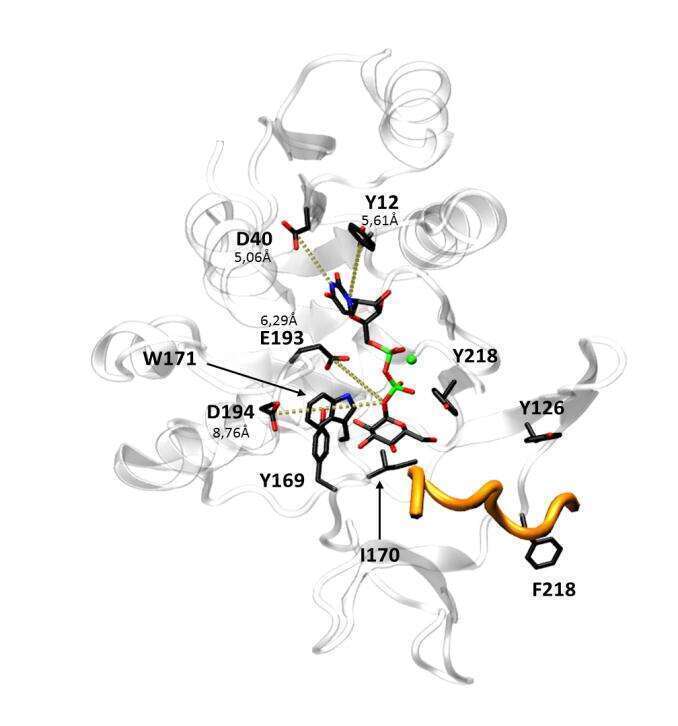
Figure1. MG517 model structure with UDP-Glucose in its active site and the residues chosen for mutagenesis highlighted.
1.Razin, S., Yogev, D., and Naot, Y. (1998)Microbiol. Mol. Biol. Rev. 62,1094-1156.
2.Lindblom, G., Brentel, I., Sjolund, M., Wikander, G., and Wieslander, A. (1986)Biochemistry 25,7502-7510.
3. Andrés, E., Martínez, N., and Planas, A. (2011) J. Biol. Chem. 286,35367-35379.

