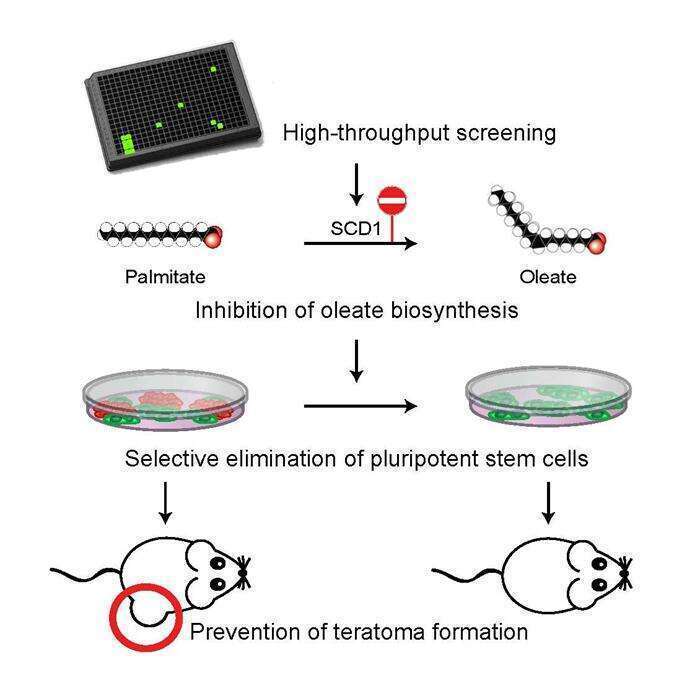
Human pluripotent stem cells (hPSCs) hold great promise for cell therapy
treatments, as they can differentiate into all the cell types of the human
body. However, the clinical use of hPSCs is hindered by the tumorigenic risk
from residual undifferentiated cells. In this study, we performed a
high-throughput screen of over 52,000 small molecules, and identified 15
pluripotent cell-specific inhibitors (PluriSIns), 9 of which share a common
structural moiety. The effect of the PluriSIns is extremely selective, as they
eliminate hPSCs rapidly and robustly while completely sparing a large array of
progenitor and differentiated cells of all germ layers and developmental stages.
Cellular and molecular analyses demonstrated that the most selective compound,
PluriSIn#1, induces ER stress, protein synthesis attenuation, and apoptosis in
hPSCs. Further characterization identified this molecule as an inhibitor of
stearoyl-coA desaturase (SCD1), the key enzyme in oleate biosynthesis,
revealing a previously unknown unique role for lipid metabolism in hPSCs. Remarkably,
exogenous supplementation of oleate completely rescued the PluriSIn#1-induced
cell death, demonstrating that oleate depletion is the direct cause of hPSC
death following exposure to PluriSIn#1. Of note, structurally-similar PluriSIns
were found to exert their cytotoxic effect on hPSCs through the same mechanism
of action. PluriSIn#1 was also cytotoxic to mouse pluripotent stem cells and to
mouse blastocysts, indicating that the dependence on oleate is inherent to the
pluripotent state, and is evolutionarily-conserved. Finally, application of
PluriSIn#1 prevented teratoma formation from tumorigenic undifferentiated hPSCs.
This novel method to eliminate undifferentiated cells from culture should thus enable
the generation of pure differentiated cultures and increase the safety of
hPSC-based therapies.
* This work has been recently published: Ben-David et al.
Cell Stem Cell 2013.