Proteins play a key role in determining food structure and functionality, and a key challenge in this respect is to obtain control over the aggregation/association state of the proteins at mesoscale lengthscale (nm-mm). Enzymatic cross-linking is widely being investigated as a possible route for obtaining additional handles on the association state of food proteins, that may be more precise than the classical route of heat-induced aggration. Most studies focus on directly relating the chemistry of enzymatic cross-linking to the final effects on food structure and functionality. But real control over these final effects requires knowledge of the the type of mesoscales protein structures that are formed by enzymatic protein cross-linking. This is the issue that we address in this talk. For the specific case of (calcium free) alpha-lactalbumin, we investigate the mesostructures that are formed when cross-linking with a range of enzymes (microbial Transglutaminase, Horse-Radish peroxidase, Laccase, Tyrosinase). We find that some enzymes lead to "hydrophilic" and others to "hydrophobic" cross-linked products. Effects on secondary structure vary between mild changes and complete denaturation. In many cases we find that soluble cross-linked structures are very heat-stable. Concentrated dispersions of cross-linked protein ("protein nanogel dispersions") have rheological properties typically associated with polysaccharide thickeners: they are visco-elastic, shear thickening physical gels with good heat stability, in short, properties that are very different from those of typical heat-set protein gels.
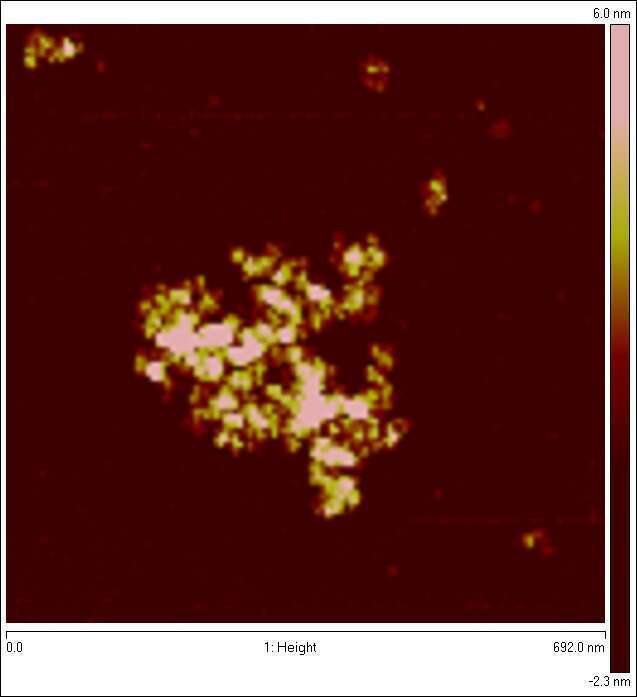
Figure 1: AFM image of dried (and flattened) protein nanogel prepared by Horse-radish peroxidase and H2O2 induced cross-linking of alpha-lactalbumin, solution hydrodynamic size is approx 100nm, internal volume fraction of protein is a few percent.
Dr. Renko de Vries renko.devries@wur.nl