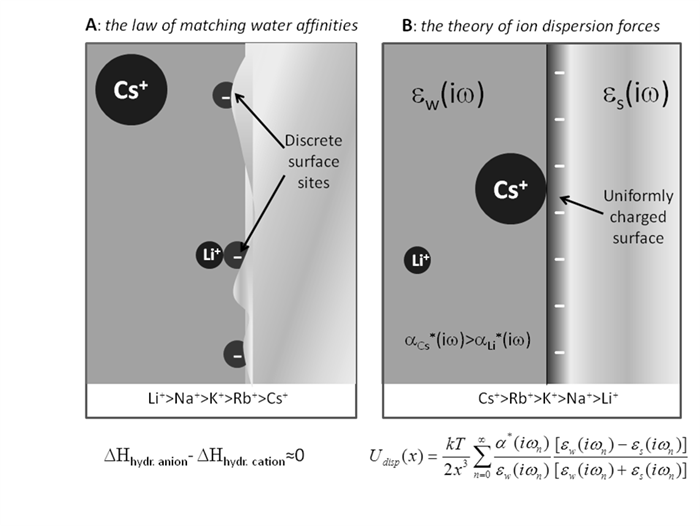
Ion specificity has posed a challenge since the 1880’s according to the pioneering work of Franz Hofmeister on salt induced protein precipitation [1]. These phenomena are ubiquitous both in chemistry and biology [2]. Conventional electrostatic theories (i.e. Debye-Huckel, DLVO) cannot explain such effects. Over the past decades it has been recognised that additional quantum mechanical dispersion forces acting on ions are missing from theory [3]. On the other hand, a phenomenological set of rules known as the law of matching water affinities (LMWA), has been proposed [4]. LMWA explains and predicts at a qualitative level the order of ion-ion and ion-surface site interactions. Ion dispersion forces and LMWA approaches appeared to conflict.
Although the need of inclusion of quantum dispersion forces is not questioned, the modeling used has sometimes not properly described the chemical nature (kosmotropic/chaotropic) of the interacting species. The success of LMWA lies in the fact that it does. Recent experimental results require that both approaches, represented in the figure, are considered depending on the particular system under investigation [5]. Here we show the way that the two apparently opposing approaches could be reconciled.
[1] W. Kunz, et al Curr. Op. Colloid Int. Sci. 9 (2004) 19-37.
[2] P. Lo Nostro, et al Chem. Rev. 112 (2012) 2286–2322.
[3] D.F. Parsons, et al Phys. Chem. Chem. Phys. 13 (2011) 12352-12367.
[4] K.D. Collins, Methods 34 (2004) 300-311.
[5] L. Medda, et al. Langmuir 29 (2013) 15350-15358.
asalis@unica.it