The formation of wormlike micelles by combining cetyltrimethylammonium bromide (CTAB) and aromatic cosolutes such as sodium salicylate is well known.1 Small changes in the structure of the cosolute affect the rheological properties of the system dramatically, such as a trans-cis isomerization of trans-orthomethoxycinnamate (OMCA).2 In this work, we have studied the rheological and calorimetric behaviors of a variety of structurally similar cosolutes, derived from cinnamate (3-phenylpropanoate, ortho-hydroxycinnamate, ortho-hydroxypropanoate, ortho-methoxycinnamate), in order to establish a logic between structure and rheology.
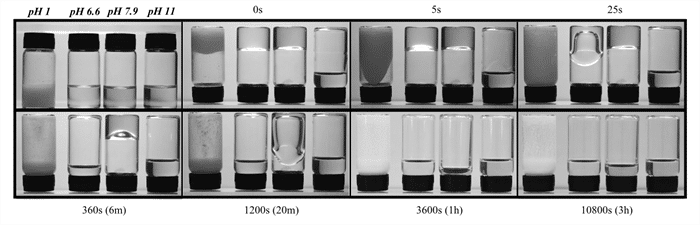
Additionally, the OHCA wormlike micelles exhibits a stark dependence on the solution pH, where a small increase in pH results on a high increase in apparent viscosity, as can be seen on the picture. Each flask has a solution of 200mM of CTAB and OHCA, on a specific pH, and the flasks were turned upside down at t=0 and then allowed to flow freely due to gravity. At pH 8, the apparent viscosity is much higher than at pH 6.6.
(1) Hoffmann, H.; Ebert, G. Surfactants, Micelles and Fascinating Phenomena. Angew. Chemie Int. Ed. English 1988, 27, 902–912.
(2) Ketner, A. M.; Kumar, R.; Davies, T. S.; Elder, P. W.; Raghavan, S. R. A Simple Class of Photorheological Fluids: Surfactant Solutions with Viscosity Tunable by Light. J. Am. Chem. Soc. 2007, 129, 1553–1559.