Unexpectedly, sessile drops of completely miscible liquids do not always coalesce immediately but can remain separated temporarily. This “non-coalescence” behavior is due to a Marangoni flow caused by the surface tension difference (∆γ) between the liquids[1],[2],[3]. When the two liquids contain reactants e.g., leading to precipitates, the drop coalescence becomes more complicated. Drop coalescence is interesting not only for fundamental aspects, but is also relevant technologically, for instance in microfluidics and chemical engineering[4].
We study a model reaction, the cerium oxalate precipitation during coalescence of drops of water/propanediol mixtures containing oxalic acid and cerium nitrate. We vary independently the reactant concentrations and ∆γ, to adjust the advection rates. We analyzed the dynamics and the precipitate morphologies by imaging and diffraction methods. In one of the identified regimes, the precipitation leads to patterns, which correspond to alternating precipitates densities and morphologies. Here we analyze the underlying mechanisms leading to the precipitation scenarios.
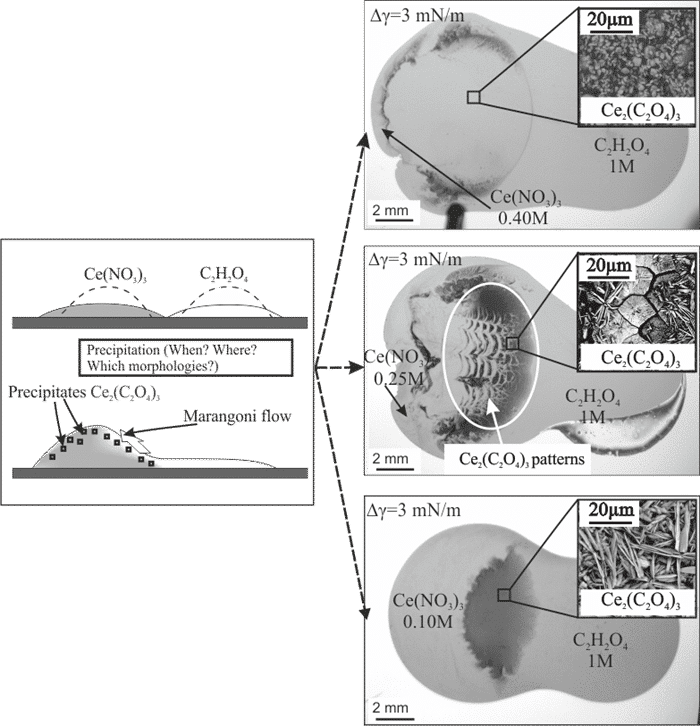
Figure 1: Left: Side view sketches. Right: Top view image of drops containing reagents precipitating after coalescence. Only the cerium nitrate concentrations vary. (Inset: SEM images of the precipitates). At intermediate Ce(N0
3)
3 concentrations, intriguing patterns are observed.
[1] S. Karpitschka and H. Riegler, J. Fluid Mech, 743, 2014, R1.
[2] S. Karpitschka and H. Riegler, Phys. Rev. Lett., 109, 2012, 066103.
[3] S. Karpitschka and H. Riegler, Langmuir, 26, 2010, 11823-11829.
[4] S. Charton et al., Chem. Eng. Res. Des., 91, 2013, 660-669.