Cellulose is the most abundant renewable material in nature that is utilized as a raw material for fabrication of synthetic products. Although it is not soluble in common solvents, there is significant interest in the use of solvent mixtures containing ionic liquids (IL) and polar organic solvents for cellulose dissolution. We present evidence for true molecular dissolution of cellulose in binary mixtures of common polar organic solvents with an ionic liquid, using cryogenic transmission electron microscopy, small-angle neutron-, x-ray- and static light scattering. In particular, the measured low values of the molecular weight (Mw), gyration radius (Rg) and persistence length indicate the absence of significant aggregation of the dissolved chains.
We conjecture that the dissolved cellulose chains are amphiphilic. This can be inferred from the facile fabrication of cellulose-encapsulated colloidal oil-in-water or water-in-oil dispersions. This may be done by mixing water, oil and cellulose solution in an ionic liquid. A more practical alternative is to form first a hydrogel from the cellulose/ionic liquid solution by coagulation with water and applying it to sonicated water/oil or oil/water mixtures. Apparently the dissolution/regeneration process affords higher mobility to the cellulose molecules so an encapsulating coating can be formed at the water-oil interface.
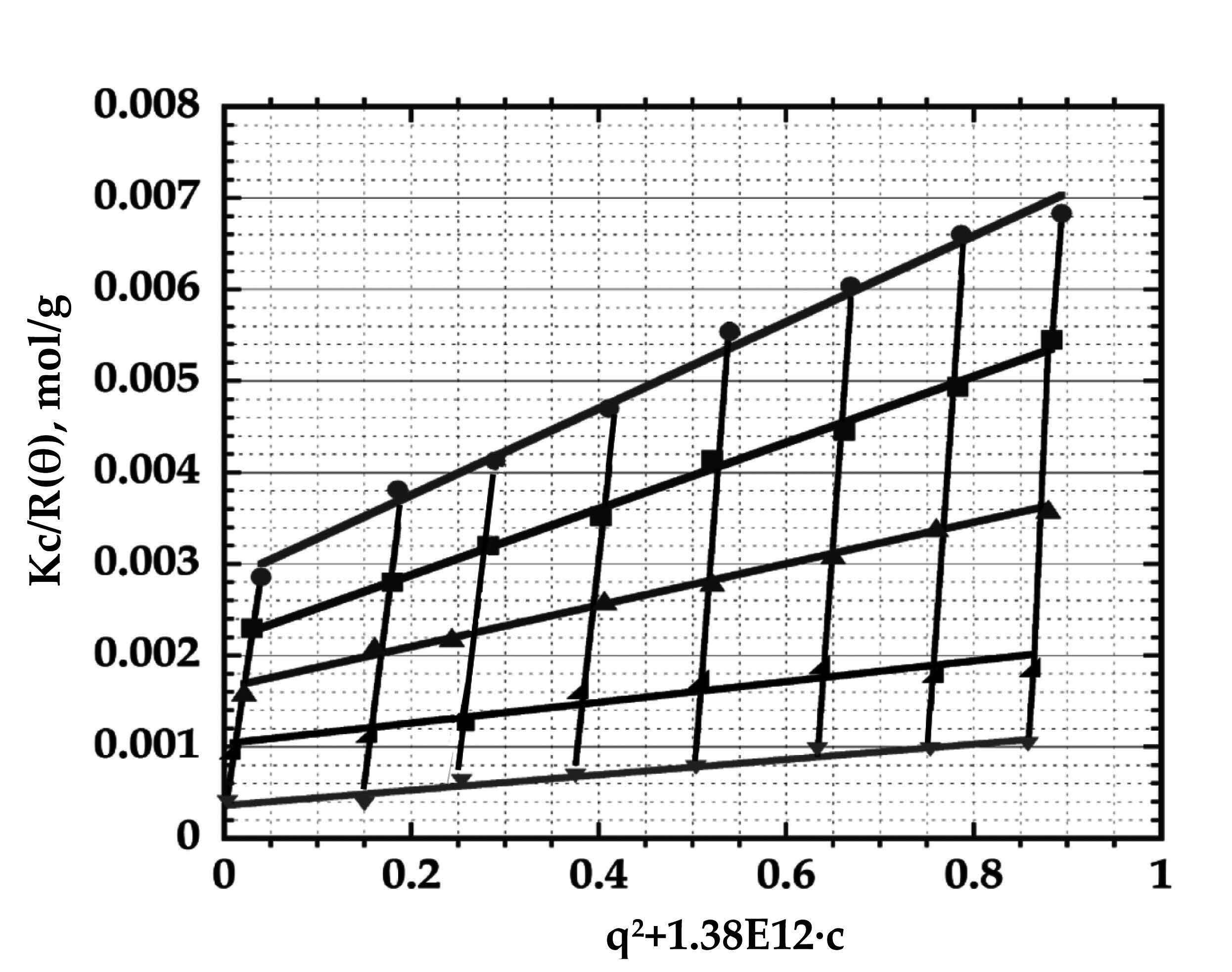
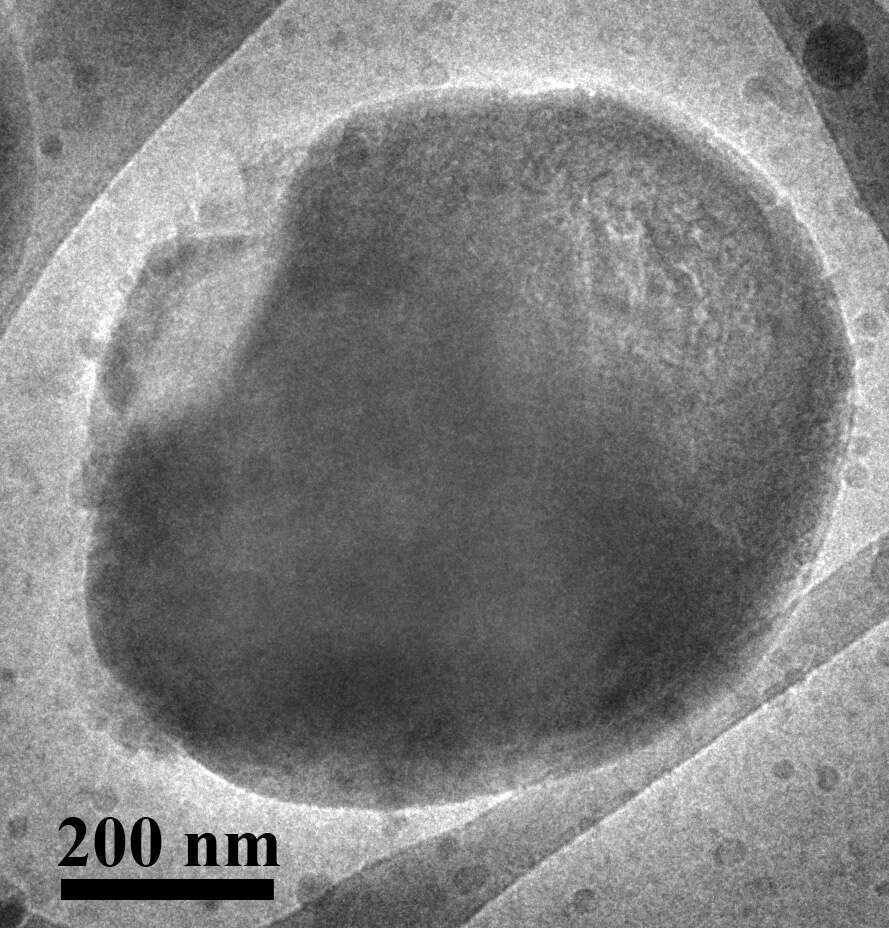
Fig. 1. Zimm plot of cellulose solutions in N-dimethyl formamide/1-ethyl-3-methylimidazolium acetate (9:1 molar ratio), yielding molecular weight Mw ~ 50 kDa, Rg ~ 36nm and second virial coefficient А2 ~ 0.025 mol·ml/g2
Fig. 2. shows a cryo-TEM image of a vitrified thin film of an aqueous dispersion of cellulose-coated paraffin oil.
yachinc@tx.technion.ac.il