Mesoporous silica can be synthesised with different properties and pore structures [1]. Some properties appear to be dependent on anions present during the synthesis for the cationic system MCM-41 [2]. In an attempt to unravel the significance of the initial interactions between the cationic micelles (the structure directing agent), silica and counterions/anions we designed a model system. The system consists of a small surfactant, C10TA+ [CH3(CH2)9N(CH3)3] with Cl-, NO3-, SO42- or para-toluene sulphonate (PTS), as counterions, and a small silica species (POSS). The anions were chosen to extend over the Hofmeister series. POSS, a cubic silsesquioxane, [Si8O20(N(CH3)4)8] forms discrete, negatively charged species in aqueous solutions with tetramethylammoniumions as counterions. This system was chosen to represent an initial snapshot of a mesoporous silica material synthesis.
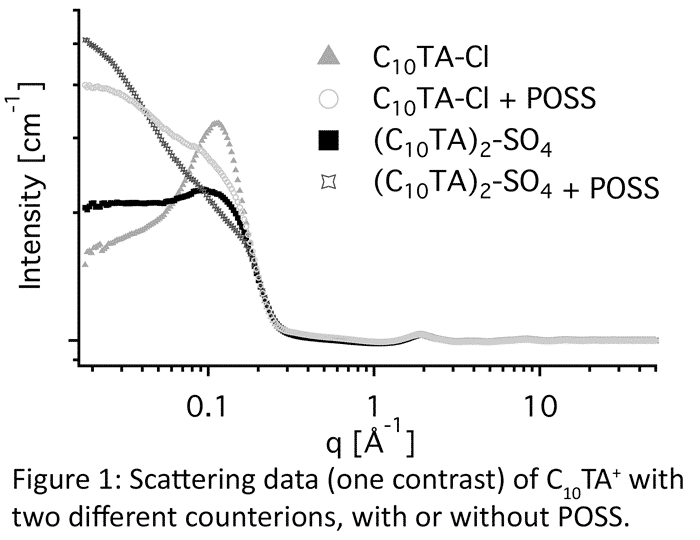 Using a combination of wide q-range neutron scattering and computer modeling [3], Empirical Potential Structure Refinement (EPSR), atomistic details of the system are provided. Our scattering data shows that different anions affect the micellar properties and that POSS interacts with the micelles. Further, this interaction depends on the identity of the anions, see Figure 1.
Using a combination of wide q-range neutron scattering and computer modeling [3], Empirical Potential Structure Refinement (EPSR), atomistic details of the system are provided. Our scattering data shows that different anions affect the micellar properties and that POSS interacts with the micelles. Further, this interaction depends on the identity of the anions, see Figure 1.
[1] Kresge, Leonowicz, Roth, Vartuli, Beck, Nature,1992, 359, 710
[2] Edler and White, Chem. Mater., 1997, 9, 1226
[3] Hargreaves, Bowron, and Edler, J. Am. Chem. Soc., 2011, 133, 16524
emelie.nilsson@fkem1.lu.se