For many applications, paper surfaces needto reduce penetration and wetting of liquids, most prominently water. This isoften achieved by surface sizing, i.e. by adsorbing a hydrophobic polymerparticle suspension on the paper surface (1). In the paper making process, theparticle suspension is combined with starch and then the mixture is applied onthe paper surface in the dry-end of the paper machine. It is important, infirst place, to understand the interactions between the different componentsused and how they influence the sizing performance. The interaction study hasfocused on the colloidal behavior of the combination of hydrophobic particlesof different charges and anionic starch. It was found that the cationicparticles form large aggregates with the starch as seen in Fig.1 where atfirst, the addition of starch to the cationic particle suspension induces anincreased turbidity due to destabilization of the system. The stability of thesystem is restored at higher starch concentrations and the particles have thenformed stable aggregates with the starch. The amphoteric and anionic particlesare not affected by the starch addition, however. The effect of ionic strengthwas also investigated.
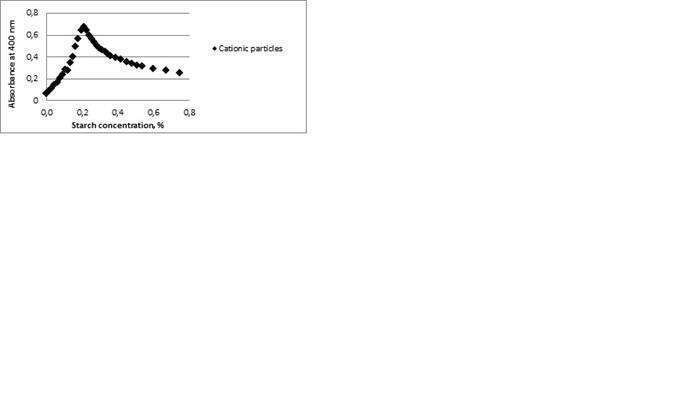
Figure 1.Turbidityplot of the cationic particle suspension at different starch concentrations.
References:
1. Ning Yang, Yulin Deng, Journal ofApplied Polymer Science, Vol.77, 2067-2073 (2000)
Frida.iselau@chalmers.se