The classical textbook picture of electrolytes at the air-water interface is shaped by surface tension measurements. Usually ions increase the surface tension, which is then interpreted within the framework of Gibbs Thermodynamic as a zone of depletion. This picture was challenged in the last decade by mounting evidence that ions may reside at the interface and moreover adopt a non-monotonous concentration profile.
In this contribution we investigate the large and easy polarisable ion hexacyanoferrate [Fe(CN)6]4- by IR-VIS sum frequency generation (SFG) spectroscopy and surface tension measurements. The equilibrium surface tension increases in a monotonic fashion with the bulk concentration. Due to selection rules a prerequisite for a second order nonlinear response is the simultaneous IR and Raman activity of vibrational modes. This implies the limitation to non-centrosymmetric media. Therefore, IR-VIS sum frequency spectroscopy provides exclusive spectra of the surface. Since the unpertubated [Fe(CN)6]4- belongs to the point group Oh, no vibrational mode is SFG active, unless a deformation takes place. A stretch of the ion reduces the symmetry to point groups, which have SFG active modes. The resulting spectra provide evidence for the symmetry breaking induced by surface effects.
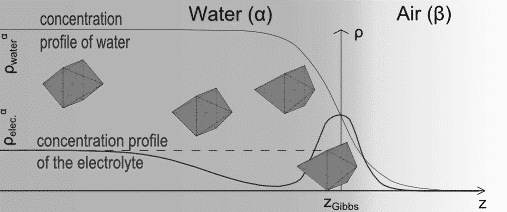
Fig. 1: Schematic representation of the distortion of the octahedral symmetry of hexacyano ferrate at the air water interface due to surface effects. A nonmonotonous concentration profile of the anion is expected.
Fig. 2: The spectra shows the SFG active modes of the CN-stretch of [Fe(CN)6]4-.This implies that the ion has no longer the octahedral symmetry.
eva.brandes@chemie.uni-regensburg.de