The interactions between proteins and particles are important for many applications, ranging from nanoparticle toxicity to sensors and material biocompatibility. Indeed proteins can either adsorb on particles, followed by structural, dynamic and functional changes [1], or remain free in solution. To investigate this process, we use the “coffee-ring” effect of evaporating drops [2] as a straightforward approach to detect molecular interactions at a macroscopic scale.
Two major blood proteins, hemoglobin and serum albumin, are mixed with polystyrene particles of different sizes and surface charges. 0.8 µL drops of various protein/particle ratios are deposited on a glass substrate and we analyze the deposition pattern after drop evaporation. A marked transition from a coffee-ring to a disk pattern is specifically observed when proteins adsorb on particle surface, thus constituting a macroscopic signature of the molecular protein/particle interaction. Moreover, for an excess in protein concentration, new deposition patterns (e.g. double-ring) are observed and can be interpreted by considering the behavior of proteins at solid/liquid and liquid/gas interfaces [3].
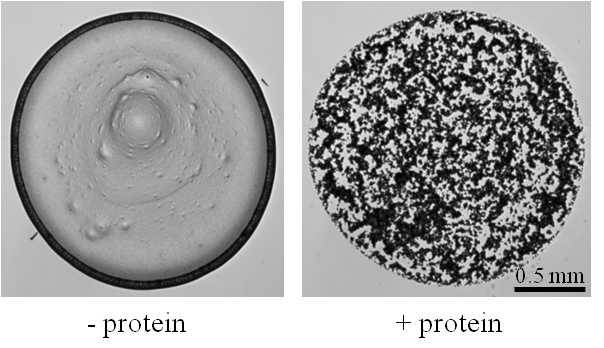
Figure. The presence of proteins dramatically changes the deposition pattern of an evaporating drop containing particles from a coffee-ring pattern (on the left) to a disk pattern (on the right). The solution contains negatively charged polystyrene particles (360 nm in diameter) without (left) and with (right) serum albumin.
References
[1] Devineau et al., Langmuir, 29, 13465-13472 (2013)
[2] Deegan et al., Nature, 389, 827-829 (1997)
[3] Devineau et al., submitted
stephanie.devineau@ens.fr