Various halogenated hydrocarbons are widely used for fire extinguishing. Particularly, carbon tetrachloride, known as Halon-104, in spite of its noticeable toxicity and ozone-depleting properties is still considered to be a perspective flame and detonation suppressant for unmanned facilities due to its quite high pyrolysis endothermicity and effective inhibiting of chain combustion reactions. It was noted in several works, though, that under specific conditions (particularly at elevated temperatures) haloalkanes may lose their inhibiting properties and even significantly accelerate ignition and flame propagation due to chemical activity of their pyrolysis products. Thus, development of modern fire safety technologies requires thorough investigation of pyrolysis kinetic and reaction ability of used active species in a wide range of conditions.
In present work an investigation of influence of CCl4 admixture on the ignition of various combustible mixtures (H2/O2, C2H2/O2 and CH4/O2) was performed in a wide range of temperatures. Experiments were carried out in the shock tube of a standard design behind reflected shock waves at pressures 4-6 bar. Ignition was detected using common technique of registration of OH radical emission signal at wavelength 306.8 nm. The ignition of hydrogen and acetylene containing mixtures was investigated at low temperatures (900-1100 K), where CCl4 molecule can be considered as stable one. Methane ignition was observed at much higher temperatures (1200-1900 К), where CCl4 decomposes rapidly. All investigated mixtures were stoichiometric ones, diluted by argon to 20% molar concentration.
In the pilot experiments it was shown that addition of 3% CCl4 significantly increases the ignition delays and thus inhibits the combustion development in hydrogen- and acetylene-oxygen mixtures. Suggested explanation involves chain branching deceleration due to various reactions like CCl4 + O → CCl3 + ClO and CCl4 + H → CCl3 + HCl where active O and H radicals are consumed. More detailed analysis of ignition suppression kinetics and modeling results will follow in a full paper.
Ignition delays in methane-oxygen mixture were, in contrast, dramatically reduced in presence of CCl4. Ignition acceleration is significant already for 0,5% CCl4 admixture and for 3% CCl4 it reaches an order of magnitude; following increasing of CCl4 concentration up to 10% doesn’t have noticeable additional influence on ignition dynamic. Obtained experimental results are shown at fig. 1 as dots.
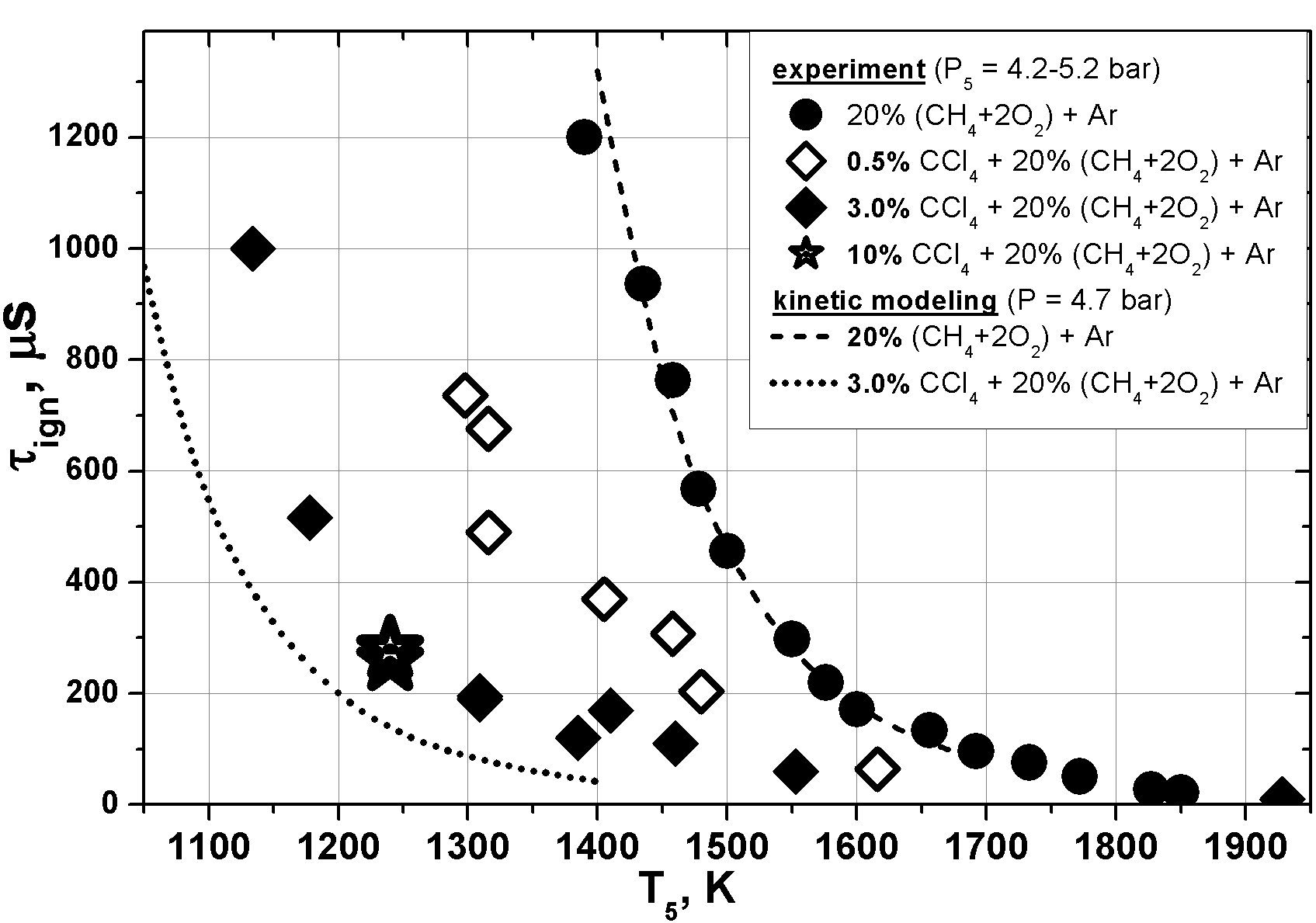
Methane-oxygen mixture ignition was modeled using well-known GRI-Mech 3.0 kinetic scheme. Kinetic model of CCl4 pyrolysis was developed and validated in recent experiments [Schulz 2013]. Main channel of CCl4 decomposition and chlorine release at considered temperatures is CCl4 → CCl3 → CCl2 → C2Cl2 → C2. Due to high complexity of both pyrolysis and combustion mechanisms, the consideration was limited by reactions involves major species as the reagents. The performed analysis indicated that promoting effect is caused by released atomic chlorine which interacts with methane in reaction where quite active methyl CH3 radical is produced. Competing chain-breaking reactions with chlorine species under the given conditions appears to be less effective.
Modeling results are shown at fig.1 as lines. One can see that quite good agreement between experimental and modeling results is obtained for methane combustion, and in presence of CCl4 admixture modeling tends to exaggerate promoting effect. Possible reasons include uncertainties of chlorine species decomposition rates (especially for reaction CCl3 → CCl2 + Cl) and known lack of reliability of combustion kinetic mechanisms at low temperatures region. Obviously, such integrated measurements as ignition delays couldn’t be used for the validation of particular reaction rates. Nevertheless, suggested kinetic mechanism demonstrates a reasonable coincidence with experimental results and describes, particularly, saturation of promoting effect at CCl4 concentrations higher than 3-4%.
One should also note that, in contrast to ignition of acetylene-oxygen mixtures, the acetylene self-decomposition and oxygen-free detonation, recently studied in [Eremin 2014], is also dramatically accelerated in presence of CCl4 at temperatures 1100-1400 K. In that case the acceleration of the process is attributed to atomic chlorine release and C2H radical formation in reaction C2H2 + Cl → C2H + HCl, which subsequently initiates the chain decomposition of acetylene. Thus, one can conclude that chemical stability of CCl4 molecule is quite essential for successive chemical suppression of ignition under the given conditions. As the temperature rises, one can expect a reversal effect of shock-wave induced combustion development promotion by released atomic chlorine. Therefore the thorough analysis and necessary precautions should be made during designing of fire extinguishing systems using carbon tetrachloride as an active species.
[Aghsaee M., Drakon A., Eremin A., Dürrstein S.H., Böhm H., Somnitz H., Fikri M., Schulz C. // Phys. Chem. Chem. Phys. 2013 Feb 28, 15(8):2821-2828]
[Drakon A., Emelianov A., Eremin A. // Shock Waves 2014, 24:231-237]

