Investigation of combustion of various mixtures is still a hot topic concerning the increase of utilization of various biofuels [1] as well as replacement of fire suppressants [2] which are able to climate change and produce environment pollutions. Experimental research of ignition and combustion processes of both simple substances such as CH4, C2H2 and real fuels mixed with air or oxygen is generally included only the measurements of temperature dependences of ignition delays and time-histories of reactants, transient radicals (OH, CH3), stable intermediates and combustion products (CO2, H2O). The time temperature behavior during ignition and combustion is important as additional significant integral characteristic for validation and refinement of detailed reaction mechanisms. The aim of this study was the development of emission-absorption spectroscopy technique for time-resolved temperature measurements in the zone of combustion of different fuels in standard shock tube experiments.
The experiments were performed behind reflected shock waves (RSW) in a conventional diaphragm type shock tube with an inner diameter of 50 mm. The initial temperature T5 and the pressure P5 behind the front of the RSW were determined based on measured incident shock wave (ISW) velocity by applying one-dimensional gas-dynamic theory with the assumption of “frozen” reaction conditions. An inaccuracy of the temperature T5 calculation was about 1-1.5 % for all range of experiments and was caused by an uncertainty of ISW velocity measured by three pressure transducers.
The temperature measurements during the reaction time were performed by line reversal spectroscopy technique. This method is based on simultaneous detection of emission and absorption of reactive mixture at the same wavelength. The measurements were carried out by two identical optical channels that focus the light from the probe region via the pair of the calcium fluoride windows installed in a horizontal plane of the shock tube and further via lenses onto two photomultipliers (Fig. 1). The first channel registered an emission only. The second channel was exposed to radiation from reference source of known brightness temperature. Thus the second channel detected the combination of absorption and emission of reaction mixture. Taking into account Lambert-Beer’s and Kirchhoff’s laws one can get the expression for the temperature determination [3]. The main advantage of this temperature measurement technique is that it requires only a calibrated light source, without knowledge of either optical properties of observable reaction mixture or the spectral sensitivity of the detection system. The tungsten ribbon lamp was used as the reference source for absorption channel. To avoid the possible influence of overequilibrium radiation of excited radicals and molecules forming in combustion reactions the wavelength of 589 nm (centered using a band pass filter with FWHM 20 nm) corresponding to sodium D-line was chosen. The sodium atoms are the inherent natural impurity in argon and in general they should be in the thermal equilibrium with the integral temperature of the mixture.
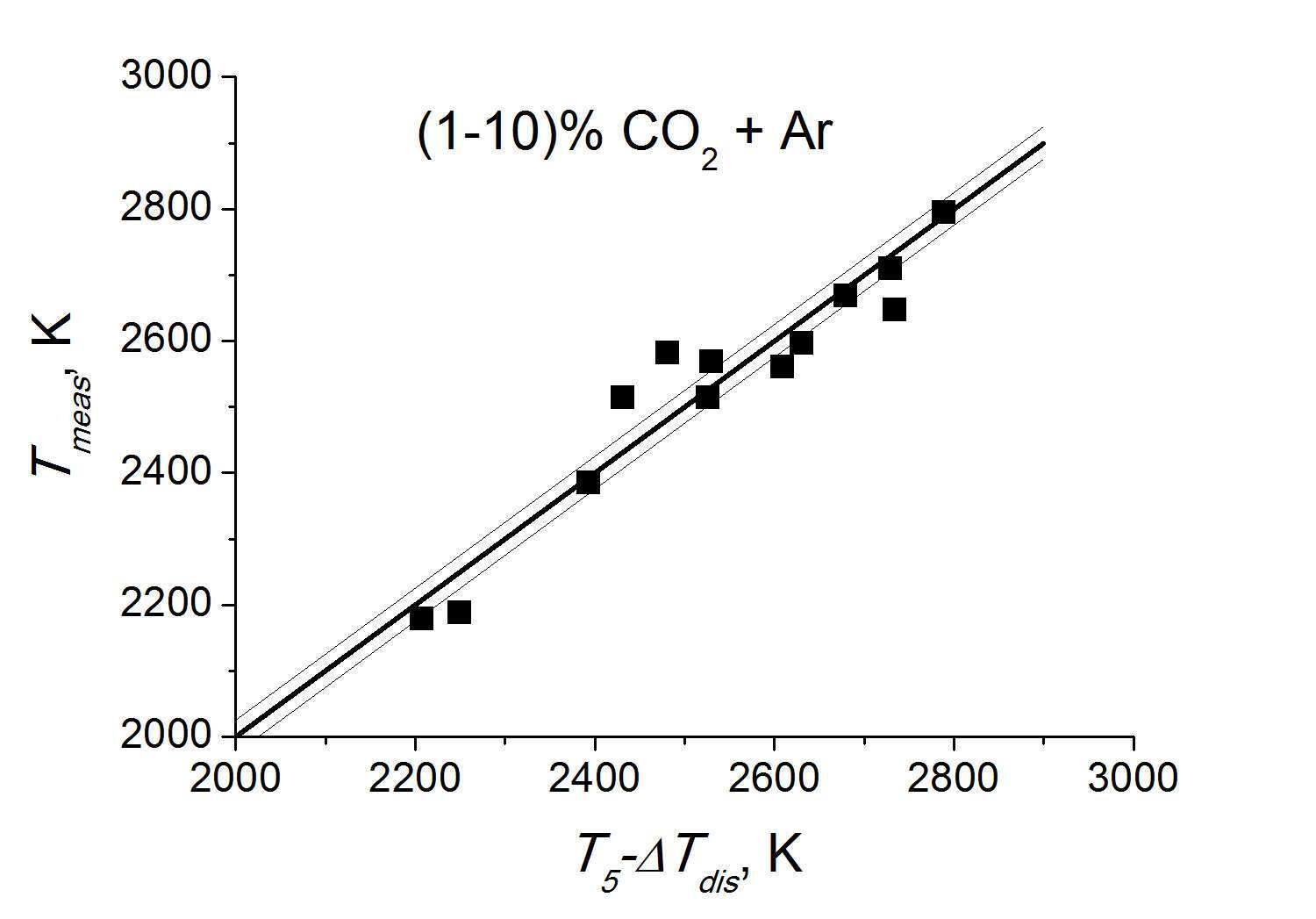
The test experiments with the non-reactive mixtures of (1-10) % CO2 + Ar in the initial temperature range of T5=2200-2800 K (see Fig. 2) were performed. At these temperatures the dissociation of carbon dioxide does not exceed 5% and corresponding temperature decrease is within ΔTdis=100 K. The error of measurements depended on the signal-to-noise ratio and the difference between the temperature of reactive mixture and the brightness temperature of reference source T0. When the value of T0 was varied in the range of 2400-2600 K the total error of temperature measurements was amounted to 5 %.
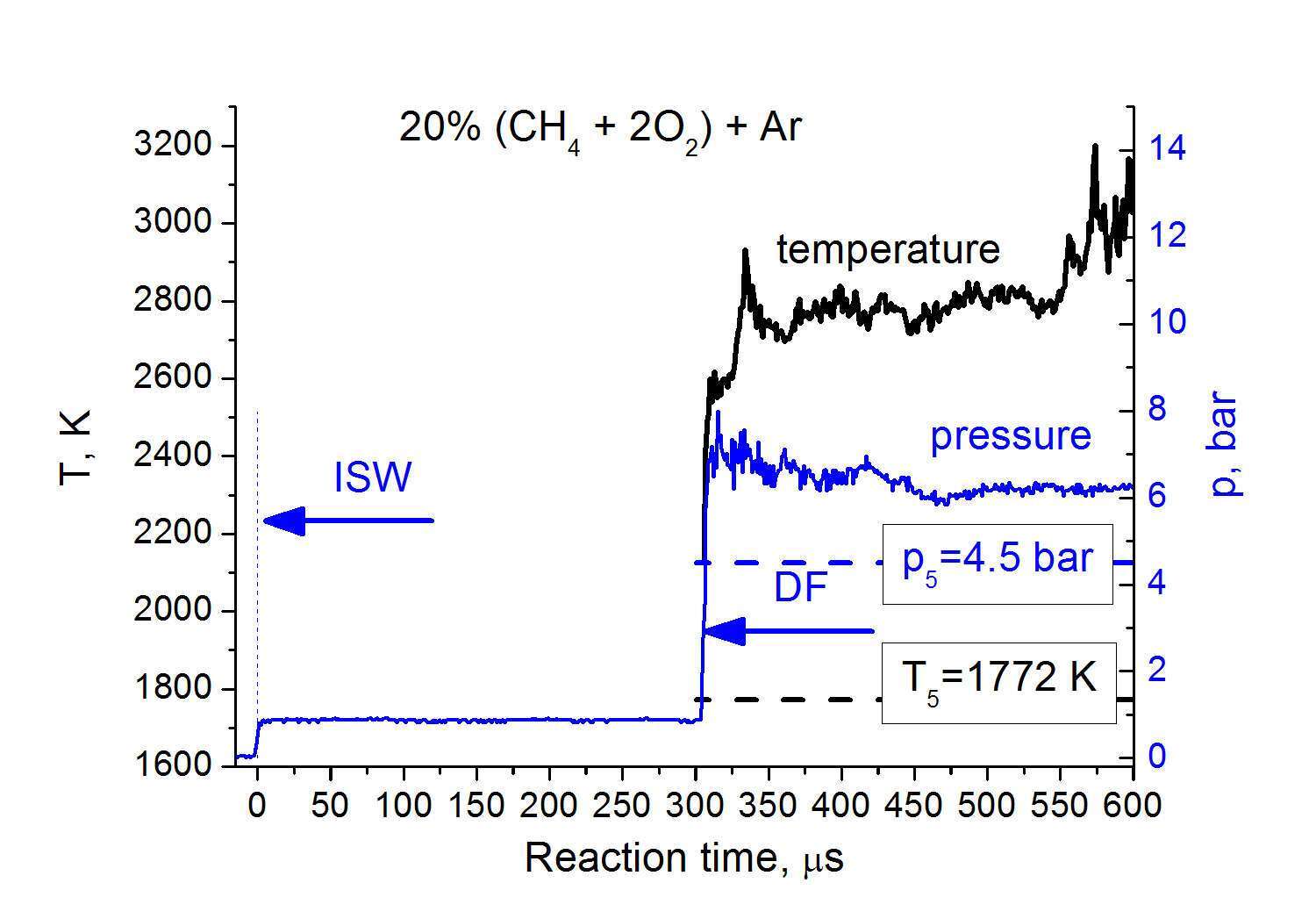
In Fig. 3 the example of measured pressure and temperature time profiles starting from combustion wave front in the mixture of 20% (CH4 + 2O2) + Ar are presented. The time scale starts with ISW arrival. The pressure behavior is the evidence that in the measurement section combustion proceeds in a detonation regime. The temperature profile shows approximately the constant value during at least 300 ms after detonation front (DF). The preliminary evaluations show a reasonable agreement of measured temperature and pressure with the Chapmen-Jouguet parameters for investigated mixture.
This work is in progress. In addition to the measurements of combustion temperature, the measurements of temperature profile in the region before the ignition are planned. For this purpose the optical scheme will be complimented by measurements in IR region of spectra.

[1] International Energy Agency. World energy outlook 2012 - renewable energy outlook; 2013. http://www.worldenergyoutlook.org/publications/weo-2013/
[2] F. Takahashi et al., Proc. Combust. Inst. (2014),
http://dx.doi.org/10.1016/j.proci.2014.05.114
[3] A. Eremin, E. Gurentsov, E. Mikheyeva, Combust. Flame 159 (2012) 3607–3615.

