The high temperature thermal decomposition of 2-phenylethanol (which is commercially used as a fragrance in perfume industry1) in argon bath has been carried out in the single pulse shock tube facility available in our laboratory2. Owing to the low volatility of the sample (2-phenylethanol ), the sample- chamber containing the sample and argon mixture has been maintained at 80ºC during the experiments in order to get sufficient sample in the vapour phase for reaction to take place . So far, the reaction mixtures have been exposed to reflected shock temperature and pressure range of 1011-1446 K and 7-13 atm respectively with the dwell times of the experiments ranging from 1.330-1.430 ms. The quantitative analyses of the pre-shocked and post-shocked mixtures have been achieved using the gas-chromatography with flame Ionization detector and gas-chromatography mass spectrometry diagnostics.
There have been two important studies3,4 with regards to pyrolysis of 2-phenylethanol (both performed using static reactors) in the temperature range 720-766 K revealing the formation of styrene, toluene, ethylbenzene as the main products. However, to our knowledge, there has been no report on the high temperature pyrolysis of this molecule so far in the literature. This has been one of the motivating factors in taking up this study to see whether any interesting observations could be made in the high temperature regime. Not surprisingly, the high temperature experiments show the formation of other additional products which were not observed in previous studies. Our study finds the formation of benzene in large abundance at high temperature in addition to styrene, toluene and ethylbenzene. Moreover Phenylacetylene and benzaldehyde are also observed as products during the analyses. This can be seen in the total-ion chromatograph shown below in figure 1.
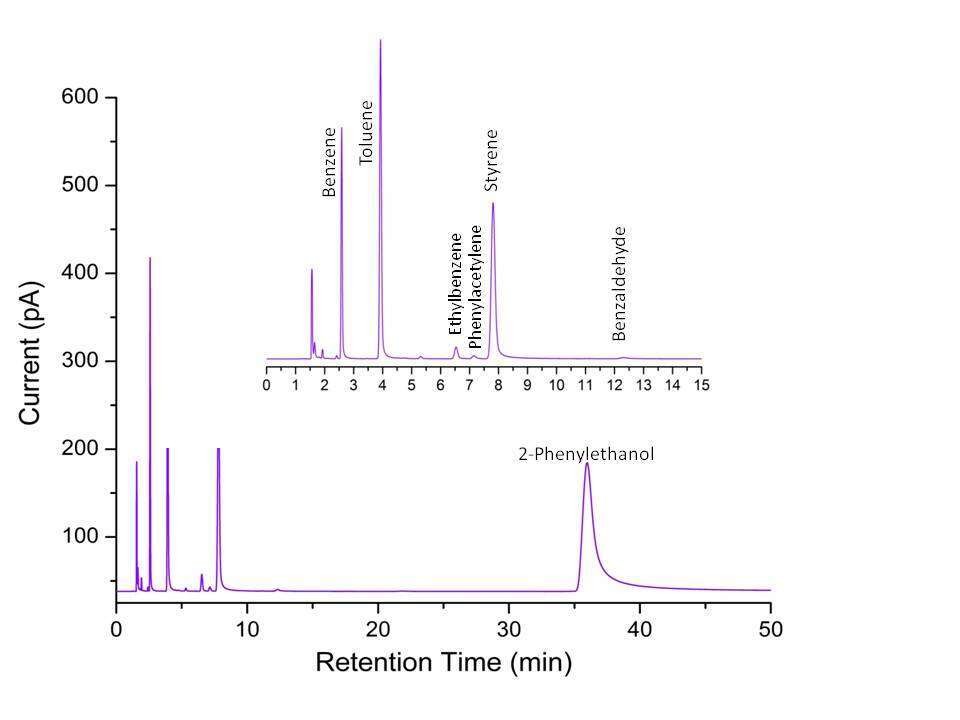
Fig. 1 Total ion chromatogram of a Post-Shock mixture of 2-phenylethanol in Argon heated to 1229K as obtained from GC-FID
The quantification of the various species in the reaction mixture is still in progress. Simultaneously, theoretical calculations based on transition state theory (TST) and Quantum theory of Atoms in Molecules (QTAIM) have also been taken up to give an in-depth explanations of the various reaction pathways involved in the experiments. Finally, a detailed kinetic mechanism using Chemkin will also be developed for the pyrolysis of the sample at high temperature. The results will be presented in the full paper.

Fig. 2 Atoms in molecules theoretical analysis of the transition state geometry for H2O elimination from 2-phenylethanol optimized at B3LYP/6-311++g(dp) level. The green dots (found between atoms) are denoted bond critical points. A ring critical point is observed in the four-center transition state involving the four atoms, two of the ethyl C atoms, H and O atoms.
References:
- Arctander, S. Perfume & FlaVour Chemicals; Montclair, NJ, 1969
- Rajakumar, B., KPJ Reddy and E. Arunan (2003) Thermal decomposition of 2-fluoroethanol : single pulse shock tube and ab initio study. J.Phys.Chem A. Vol. 107, 9782-9793
- Taylor, R. J (1988) The Mechanism of Thermal Eliminations. Part 25. Arrhenius Data for Pyrolysis of lsochroman-3-one, Benzyl Methyl Ether, 2-Hydroxyethylbenzene, Phenyl Acetate, and 3,4-Dihydro-ZH-pyran Chem Soc Perkin Trans 2, 183.
- Chuchani, G., Rotinov, A., Dominguez, R.,M. (1999) The Kinetics and Mechanisms of Gas Phase Elimination of Primary, Secondary, and Tertiary 2-Hydroxyalkylbenzenes Int J Chem Kinet 31: 401–407.

