Our experimental and modeling study of the soot formation during the pyrolysis of a number of aromatic (benzene, toluene, and ethylbenzene [1]) and saturated aliphatic hydrocarbons (methane, propane [2–4]) showed good agreement between the results of experiments and detailed kinetic modeling. However, certain difficulties were encountered in the kinetic simulation of soot formation in the pyrolysis of acetylene, a hydrocarbon with a triple bond. These difficulties have been overcome by introducing of additional reaction channels of soot nucleation from hydrogenated polyyne-like fragments, in particular, diacetylene dimers. The experiments performed in [5] confirmed the possibility of the formation of high concentrations of diacetylene dimers in the reaction mixture.
The aim of this work was to perform an experimental and kinetic modeling studies of soot formation in the pyrolysis and oxidation of various mixtures of aliphatic and aromatic hydrocarbons in argon behind reflected shock waves and to demonstrate the predictive capabilities of the modified kinetic model of soot formation.
The soot yield and temperature of soot particles were determined using a two-beam technique that enables to simultaneously measure the optical absorption and emission of soot particles. Details of the shock-tube setup were described elsewhere [3]. Figure 1 shows typical time histories of the absorption (1) and emission (2) signals after the arrival of the reflected shock wave front at the observation section, as well as the time evolution of the soot yield (3) and the soot particles temperature (4) obtained from signals (1) and (2) for the pyrolysis of a 5 % mixture of acetylene in argon under various conditions behind the reflected shock wave.
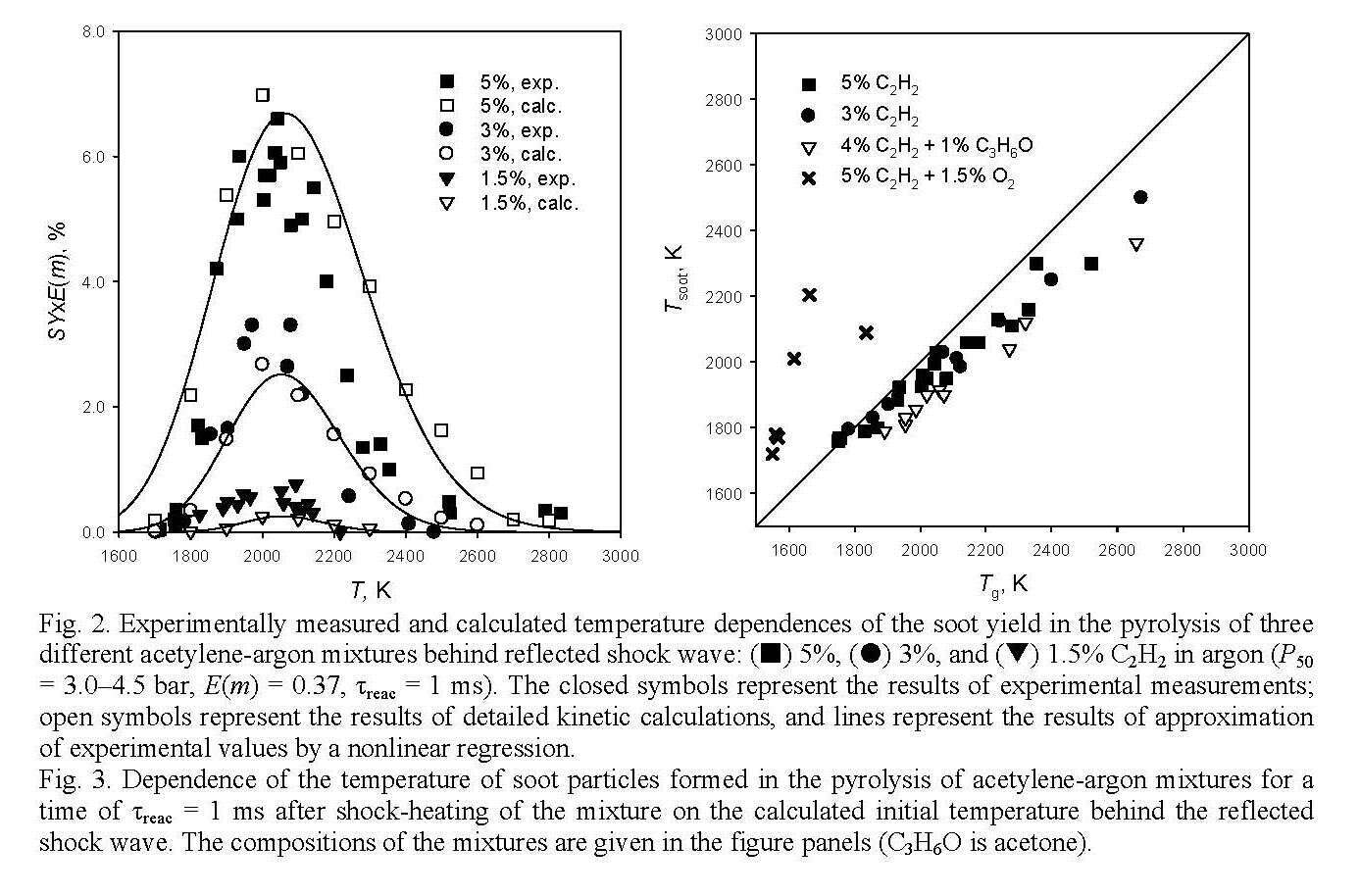
Experimentally measured dependences of the soot yield on the initial temperature of the test mixture behind the reflected shock wave for three different acetylene-argon mixtures are displayed in Fig. 2. These curves have a characteristic bell-like shape. As can be seen, the soot yield strongly depends on the initial concentration of acetylene in the test mixture. Figure 3 shows the deviation of the temperature of soot particles formed during the pyrolysis of acetylene–argon mixtures for a reaction time of 1 ms from the calculated initial temperature behind the reflected shock wave.
Kinetic simulations were performed using the mechanism of soot formation developed in [4]. This mechanism of the formation of soot particles includes a submechanism of gas-phase reaction for describing the pyrolysis and oxidation of the starting hydrocarbons, in particular acetylene, and the formation and growth of polyaromatic hydrocarbon molecules through various channels, up to coronene inclusive. The principles of constructing this kinetic mechanism were outlined in [4]. The modified gas-phase reaction mechanism consisted of 3531 direct and inverse reactions involving 300 different components, with the rate constants of several important reactions being pressure-dependent.
The processes of formation, growth, oxidation, and coagulation of soot particle nuclei and actually soot particles were simulated using the discrete Galerkin method [2, 4].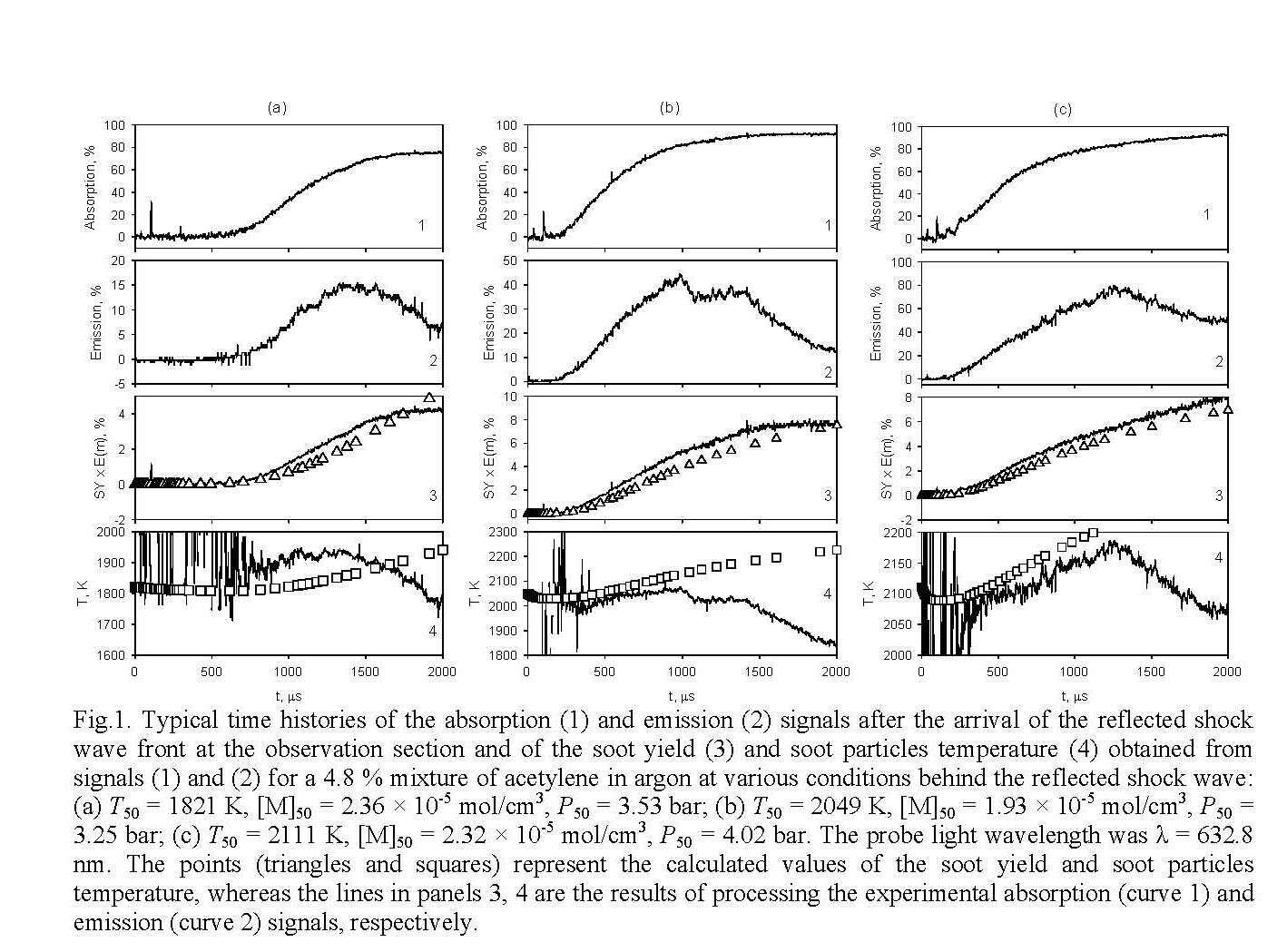
Introduction into the soot formation kinetic model of the soot nuclei produced from unsaturated aliphatic hydrocarbons, along with those arising from polyaromatic compounds, makes it possible to significantly improve the kinetic description of the experimental time histories of soot formation (Fig. 1).
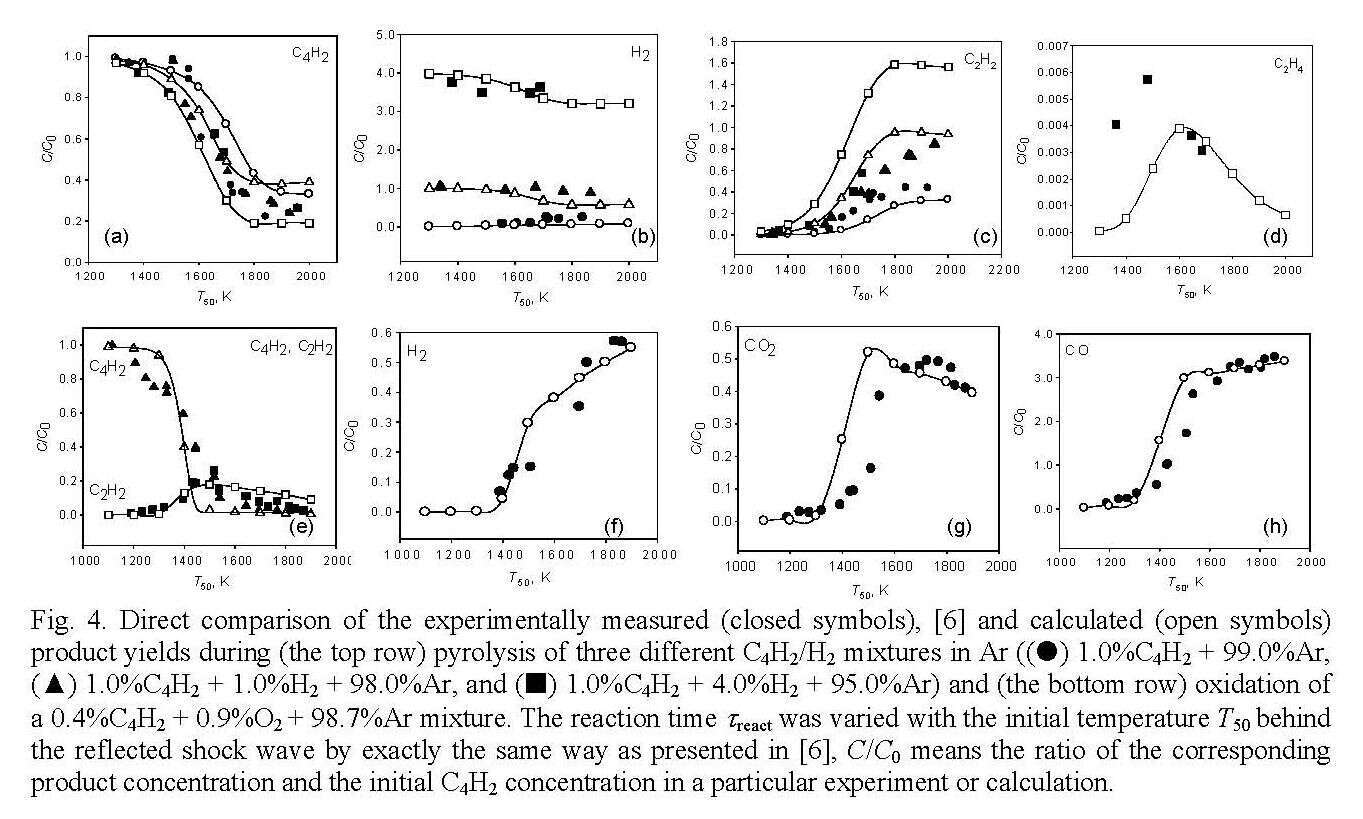
Figure 4 compares the experimental data from [6] with the results of our kinetic simulations.
REFERENCES
[1] Agafonov, G.L., Smirnov, V.N., Vlasov, P.A. (2011). Soot formation in the pyrolysis of benzene, methylbenzene, and ethylbenzene in shock waves, Kinet. Catalysis, 52(3), 358–370.
[2] Agafonov, G.L., Borisov, A.A., Smirnov, V.N., Troshin, K.Ya., Vlasov, P.A., and Warnatz Jurgen. (2008). Soot Formation during Pyrolysis of Methane and Rich Methane/Oxygen Mixtures behind Reflected Shock Waves, Combust. Sci. Technol., 180(10), 1876–1899.
[3] Agafonov, G.L., Smirnov, V.N., Vlasov, P.A. (2010). Shock tube and modeling study of soot formation during pyrolysis of propane, propane/toluene and rich propane/oxygen mixtures. Combust. Sci. Technol., 182(11), 1645–1671.
[4] Agafonov, G.L., Smirnov, V.N., Vlasov, P.A. (2011). Shock tube and modeling study of soot formation during the pyrolysis and oxidation of a number of aliphatic and aromatic hydrocarbons. Proc. Combust. Inst., 33, 625–632.
[5] Homann, K.H., Pidoll, U. (1986). The Low-Pressure Pyrolysis of Butadiyne (C4H2). Ber. Bunsenges. Phys. Chem., 90, 847–854.
[6] Hidaka, Y., Henmi, Y., Ohonishi, T., Okuno, T., Koike, T. (2002). Shock-Tube and Modeling Study of Diacetylene Pyrolysis and Oxidation. Combust. Flame, 130, 62–82.

