In continuation with our previous work an oxidation mechanism to fit the experimentally obtained ignition delay values of 3-carene has been derived1,2. The proposed reaction mechanism consists of 97 species and 250 reactions. The oxidation chemistry of radicals formed from the decomposition of 3-carene are also considered. In order to reduce the computational time the formation of peroxy radicals were considered only for C9 and lower hydrocarbons. The molecular structures peroxy radicals, which were products of O2 reaction with radicals formed in the initial steps of 3-carene decomposition process and also, used in the oxidation mechanism, has been given in Figure 1.

Figure 1: Structure of intermediates considered in the oxidation mechanism.
The constant volume method available in CHEMKIN3 has been used to simulate the mechanism. The ignition delay in the present simulation is defined as the time taken by CH mole fraction to reach its peak value for equivalence ratios 0.5 and 1. In the case of equivalence ratio 2 the computed ignition delay time was defined as the time taken for the temperature in the reactor to rise by 400 K. One of the obtained pressure profiles and CH mole fraction profile has been given in Figure 2. The comparison between experimental and computed ignition delay values are given in Figure 3.
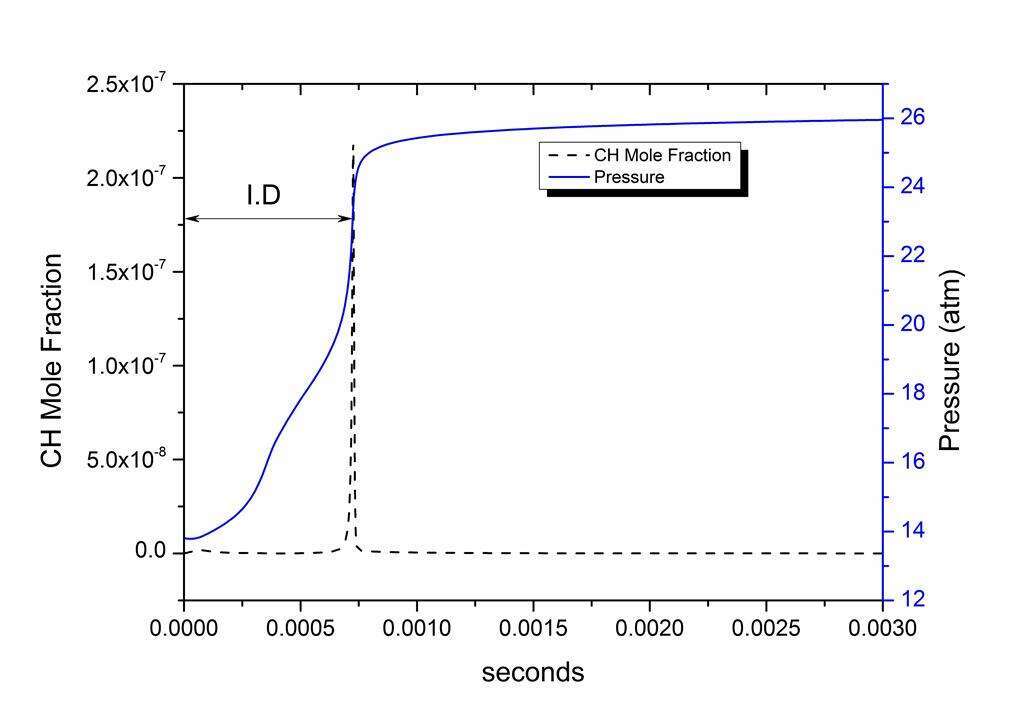
Figure 2: The pressure and CH mole fraction profiles obtained in the present simulation at 1236 K for stoichiometric 3-carene/O2/argon mixture.

Figure 3: Comparison between experimental and computed ignition delay values for equivalence ratios 1, 0.5 and 2, in cyclic order.
The sensitivity analysis, which was carried out to understand the importance of reactions in the mechanism showed that the reactions involving O and OH radicals plays an important role propagation. The work on fine tuning the oxidation mechanism is in progress and the detailed results will be given in full length paper.
References
1.Sharath N, Reddy K.P.J and Arunan E, Oxidation of 3-carene at high temperature, 28th International Symposium on Shock Waves, Edited by Konstantinos Kontis.
2.Sharath N, Reddy K.P.J and Arunan E, Thermal decomposition study of 3-carene, ISSW-29, Madison, USA, Jul 14-19, 2013
3.CHEMKIN-PRO 15101, Reaction Design: San Diego 2010.

