Production of biofuels from biomass by chemical/biological transformations without making any impact on human or animal food supplies1 is a challenging task. Though generation of biofuel is important in one aspect, knowledge on pyrolysis and combustion studies of these biofuels is very much essential. 2-Methyltetrahydrofuran is considered as next generation biofuels and also used as an additive to gasoline. This present study is focused to investigate the combustion properties of 2-methyltetrahydrofuran.
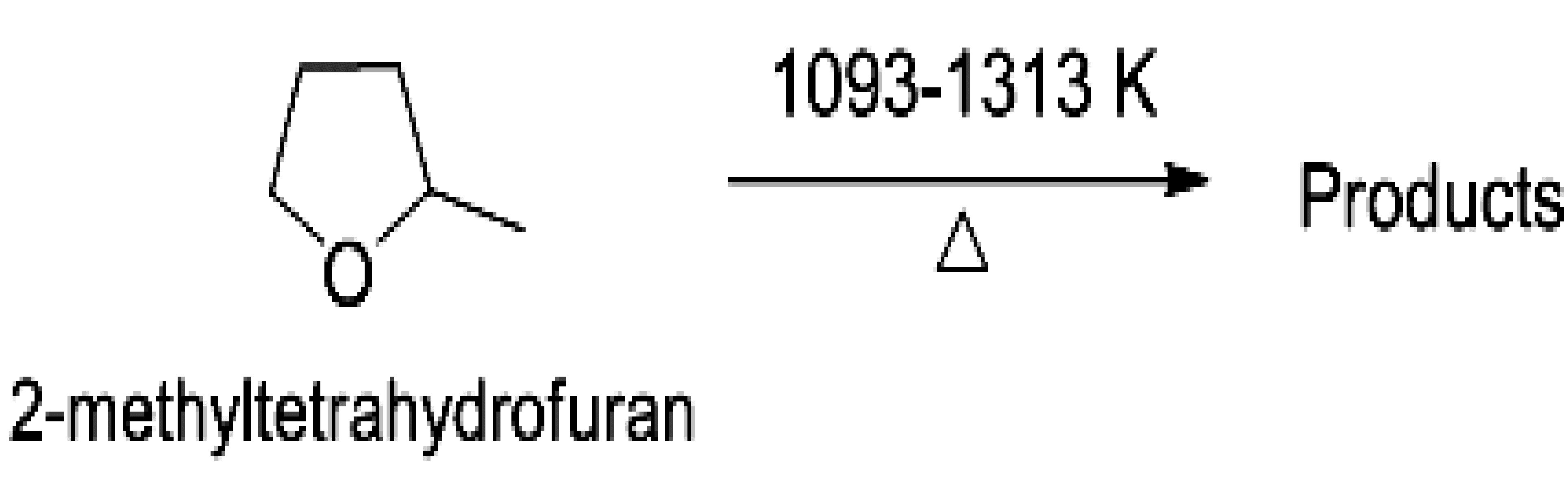
The thermal decomposition of 2-methyltetrahydrofuran (2-MTHF) was studied behind reflected shock waves in a single-pulse shock tube over the temperature range of 1093-1313 K. Two different internal standards (1, 1, 1-trifluoroethane and chlorocyclopentane) were used to estimate reliable reflected shock temperature in the reaction zone2-3. The Arrhenius expression for the total decomposition of 2-MTHF is estimated to be k (1093-1313 K) = 1.02 × 1010 (s-1) exp (-40.7 kcalmol-1/RT). The identified products are methane, ethylene, ethane, acetylene, propylene, acetaldehyde, and 1, 3-butadiene over the studied temperature range. The distribution of reactant and product concentrations over the studied temperature range and the Arrhenius behavior of the measured data are shown in Figure 1. The experimental conditions, mechanism for the formation of products and comparison of present results with simulation results will be presented in detail during the conference.

Figure 1. Concentration profiles and Arrhenius plot for the overall decomposition of 2-methyltetrahydrofuran over the temperature range of 1093-1313 K.
References:
[1]. Briens, C.; Piskorz, J.; Berruti, F. Biomass Valorization for Fuel and Chemicals Production-A Review J. Chem. Reactor Eng. 2008, 6, A111.
[2]. I. A.; Burgess Jr, D. R.; Tsang, W.; Manion, J. A. Standard reactions for comparative rate studies: Experiments on the dehydrochlorination reactions of 2-chloropropane, chlorocyclopentane, and chlorocyclohexane. Int. J. Chem. Kinet. 2012, 44, 351.
[3]. Tsang, W.; Lifshitz, A. Kinetic stability of 1, 1, 1-trifluoroethane. J. Chem. Kinet. 1998, 30, 621.

