Biofuels [1,2], consisting of long-chain alkyl (methyl, ethyl, propyl) esters, have emerged as viable alternatives to petroleum-based fuels. The biodiesel is used as an additive to fuel for two main reasons. This can reduce dependence on imported petroleum and can also contribute to environmental preservation by lowering net emissions of greenhouse gases. The use of biodiesel in diesel engines decreases emissions of pollutants/greenhouse gasses such as carbon monoxide, and unburned hydrocarbons. From a combustion chemistry standpoint, there is great interest in developing accurate reaction models for these new molecules that can be used to predict their behavior in various temperature regions.
The thermal decomposition of methyl butanoate diluted in argon have been studied behind the reflected shock waves in the temperature range of 1190-1355 K, pressures varied between 10 and 16 atm and reaction times between 450 and 900 µs using single pulse shock tube technique [3]. The post shock mixtures were analyzed quantitatively using gas chromatography (GC) and qualitatively using Fourier-transform infrared (FTIR) spectroscopic analysis. Methane (CH4), ethylene (C2H4), acetylene (C2H2), are found to be major products and propylene (C3H6), ethane (C2H6), 1,3-butadiene (C4H6), methyl acrylate (C4H6O2) are found to be minor products in the decomposition. The decomposition happens via (i) C-C bond fission, (ii) C-O bond fission and (iii) hydrogen transfer reactions.
The measured first order rate coefficient for the overall decomposition of methyl butanoate is ktotal = (2.92 ± 1.02) × 1012 exp (-(57.3 ± 2.0)/RT) s-1, and for the ethylene (C2H4) formation channel, the rate coefficient is obtained to be k = (6.71 ± 1.05) × 1010 exp (-(51.3 ± 4.0)/RT) s-1. The concentration of the reactant and products distribution over the studied temperature and the Arrhenius plot for the overall decomposition of methyl butanoate are shown in Figure 1 and Figure 2 respectively. A reaction scheme containing 27 species and 23 elementary reactions was proposed to simulate the reactant and product concentrations over the temperature range of 1190-1355 K using IBM’s chemical kinetic simulator [4] and it is given in the Table 1. The agreement between the experimental results and the model prediction for all the species is observed to be reasonably good. The details of experimental investigations will be discussed in detail in the conference.
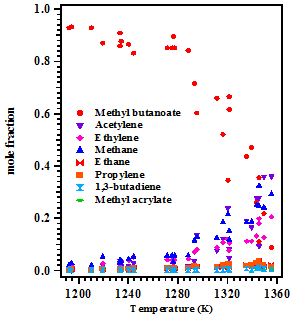
Figure 1. The concentration profiles of the reactant and product distribution in the decomposition of methyl butanoate in the temperature range of 1190-1355 K.
Figure 2. Arrhenius plot for the overall decomposition of methyl butanoate in the temperature range of 1190-1355 K.
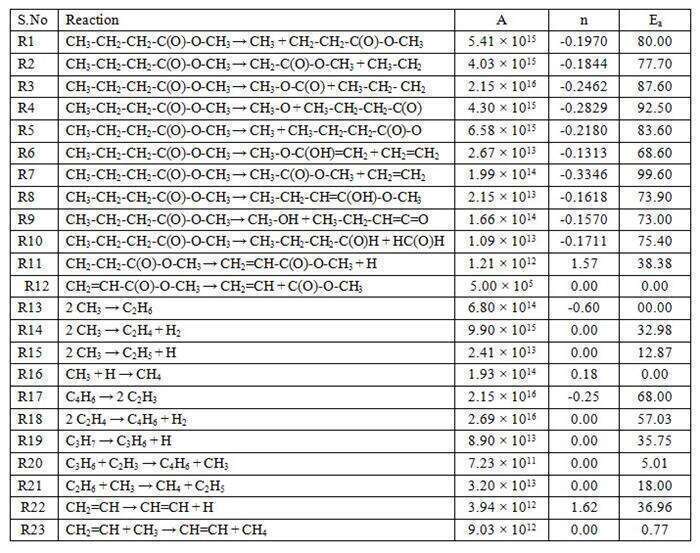
Table 1. Proposed reaction scheme for the decomposition of methyl butanoate in the temperature range of 1190-1355K.
References:
[1] Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sust. Energy Rev. 2008, 12, 542–552.
[2] Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164.
[3] Gaydon, A.G.; Hurle, I.R. The Shock Tube in High-Temperature Chemical Physics, Reinhold Publishing, New York, 1963.
[4] Chemical Kinetics Simulator 1.0. IBM Almaden research center, IBM Corporation 1995.
[5] Akbar Ali, M.; Violi, A. Reaction Pathways for the Thermal Decomposition of Methyl Butanoate. J. Org. Chem. 2013, 78, 5898−5908.

