Abstract: Light weight silica tiles are used as thermal protection systems (TPS) materials to protect re-entry space capsules. When re-entry space capsule flies at very high Mach number, dissociation of air takes place inside the shock layer. Silica tiles made out of SiO2 fibers show low catalysity when it interacts with the dissociated air. TPS materials having low catalytic property will prevent recombination of dissociated gas atoms on the surface [1, 2]. Material shock tube (MST1) facility established in the Shock Induced Materials Chemistry Laboratory, SSCU, Indian Institute of Science, Bangalore operating at a stagnation enthalpy of 2-4 MJ/kg is used to heat the test gases to high temperatures. In the present experimental investigations, shock heated nitrogen gas interacts with the SiO2 fine powder for about 1ms inside the shock tube (MST1).
The objective of this paper is to explore the possibility of understanding the non-catalytic reactions with the silicon dioxide fine powder using shock heated N2 gas. Ultra high pure nitrogen gas filled inside the shock tube with a pressure of 10 kPa is heated to about 3800 K (estimated) at reflected shock pressure of about 2.5 MPa for about 1 ms. High temperature N2 gas interacts with the SiO2 fine particles and forms silicon oxynitride particles, which is the signature of non-catalytic reactions. The surface morphology and composition of the shock heated SiO2 fine particles before and after exposure to shock heated N2 were examined using different experimental techniques like powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR).
XRD of unexposed SiO2 powder shows the mixture of both cristobalite (JCPDS number 01-077-1316) and α-quartz (JCPDS number 01-078-1252) crystal structures as shown in Fig.1a. XRD of exposed SiO2 fine powder, shows the formation of silicon oxynitride Si2N2O (JCPDS number 00-47-1627) compound after one shock and two shocks treatment, as shown in Fig. 1b and 1c, respectively. XPS analysis shows the formation of silicon oxynitride powder, when SiO2 powder reacts with shock heated nitrogen gas. Deconvoluted XPS spectra of Si (2p) show peaks at 103.9 eV due to the formation of silicon oxynitride, and at 103.3 eV due to SiO2 powder (figure not shown). The details of the experimental arrangement and the results will be presented in the full length paper.
Acknowledgements: Financial support for this research work from STC, DRDO, JATP and DST is gratefully acknowledged.
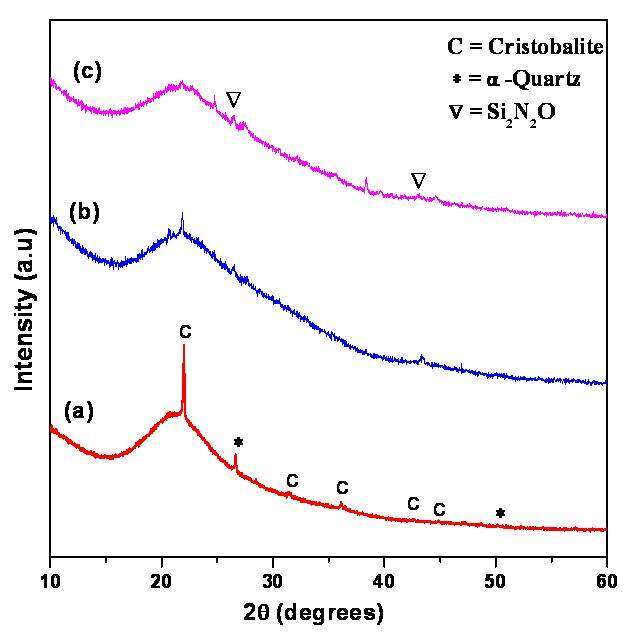
Fig.1: XRD of SiO2 fine powder (a) before shock treatment shows mixed phase of cristobalite and α- quartz, (b) after 1 shock and (c) after 2 shocks in presence of N2 gas
Keywords: SiO2 powder, TPS materials, Material Shock Tube, Surface nitridation, Non-catalytic reaction
References:
[1] Shuichi Ueda, Matthias Weiland, Katsuhiro Itoh, and Klaus Hannemann: Comparative Experiment on the Surface Catalysity in Two High Enthalpy Shock Tunnels. 24th AIAA Aerodynamic Measurement Technology and Ground Testing Conference, Portland,Oregon, 28th June - 1st July (2004)
[2] K. P. J. Reddy, M. S. Hedge, and V. Jayaram: Material processing and surface reactionstudies in free piston driven shock tube, 26th International Symposium on Shock Waves, Gottingen, Germany, (Plenary talk), Page 35-42, 15-20 July (2007)

