Objective
The Bicuspid aortic valve (BAV) is associated with valvular pathologies such as aortic regurgitation and aortic stenosis. Possible cause for these complications is related to a non-optimal geometry of the valve. Common configuration of BAV has one raphe with varying cusps angles. Surgical repair is often performed in BAVs with smaller non fused cusp (NFC) angle.
This study aims to provide a new parametric biomechanical modeling approach of BAVs to investigate the influence of material and geometrics factors on its functionality. Effect on parameters such as effective orifice area (EOA) and stresses magnitudes on the tissue and raphe are examined.
Methods
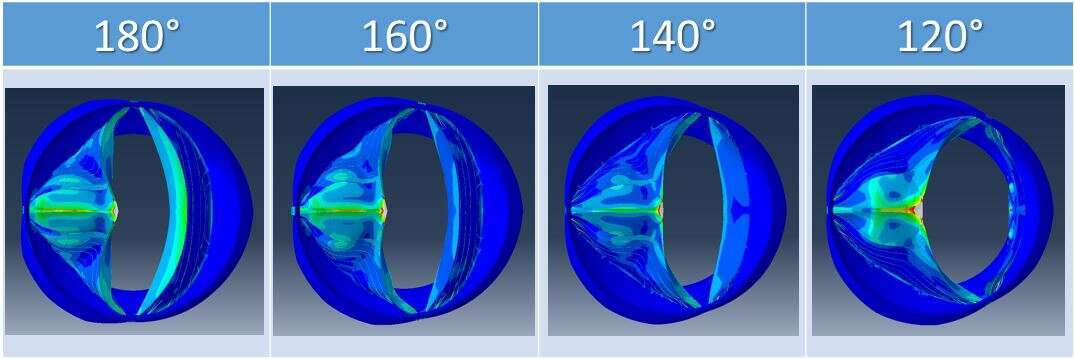
Parametric 3D numerical finite-element (FE) models of BAVs were generated, with four common BAV geometries, these include one raphe, and different NFC angles, varies from 120° to 180°. Material properties of the structure and the applied transvalvular pressure corresponded to physiologic values. Echocardiography (Echo) data from patients with BAV was used to correlate with the present FE models results.
Results
The simulated BAVs have significant geometrical differences leading to different mechanical responses and behavior. A decreased and centrally located effective orifice areas (EOAs) were found in the models having increased NFC angles. The measured echo results support this predicted pattern. During peak systole, the stresses on the raphe increase linearly as function of the NFC angles. Similarly during diastole, high stresses were found on the NFC with the decreased angle, which can explain the early failure in BAVs with smaller NFC angles.
Conclusions
The predicted biomechanical behavior in the form of stresses and kinematics can help assess the functionality and durability of BAVs. High stress regions on the leaflets can help explain the initiation of calcification. The present computational modeling is a step forward towards patient specific simulation of future BAV repair.