Background: Inflammation leads to macrophage accumulation in unstable atherosclerotic plaques, which eventually weakens the extracellular matrix and causes plaque rupture. Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme produced by inflammatory cells, co-travels with circulating low-density lipoprotein (LDL), and hydrolyzes oxidized phospholipids in LDL. The product of Lp-PLA2 are bioactive lipid mediators, which are generated in lesion-prone vasculature, and are known to elicit inflammatory responses. HDL is known for its anti-atherosclerotic properties through various mechanisms, including inhibition of Lp-PLA2. We hypnotized that gold nanoparticles which are scavenged by macrophages, can be used in order to deliver HDL to inflammatory plaques and thereby reduce inflammatory activity.
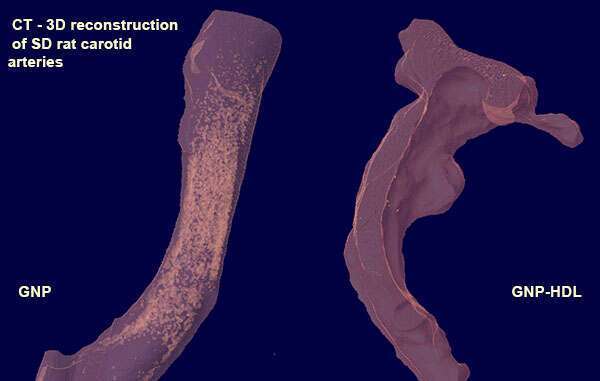
Methods: Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy blood donors. The cells were allowed to differentiate into macrophages (validated by immunostaining) and were then incubated with GNP and GNP conjugated to HDL. Lp-PLA2 levels were measured in the supernatant using ELISA. The hypothesis was tested in an in-vivo model of injured carotid artery in Sprague-Dawley(SD) rats. The animals were injected with HDL conjugated GNP and were compared to GNP alone. Atherosclerosis was evaluated through immunostaining and CT (Figure).
Results: Lp-PLA2 level of the GNP-HDL was lower compared to control 0.0125±0.001 pg/ml vs 0.0134 ±0.001 pg/ml (P=0.03). There were no significant differences between GNP to GNP-HDL or between GNP and Control. Preliminary results in the rat model showed that HDL conjugated GNP halted the inflammatory process compared to GNP control.
Conclusion: Gold nanoparticles can serve as a carrier for anti-atherogenic treatment. The use of GNP conjugated to HDL was shown to decrease the level of Lp-PLA2 a pro-atherogenic factor in the supernatants of human macrophages. These in-vitro finding should be further validated in the in-vivo animal model.