Background Transcatheter aortic valve implantation is the contemporary treatment of choice for high/prohibitive surgical risk patients with severe symptomatic aortic stenosis.
Methods Outcomes of 329 (57% female) severe aortic stenosis patients, treated with TAVI and followed up to 3 years (mean 886 days), were analyzed and reported according to the Valve Academic Research Consortium 2 definitions. The primary end point was death from any cause.
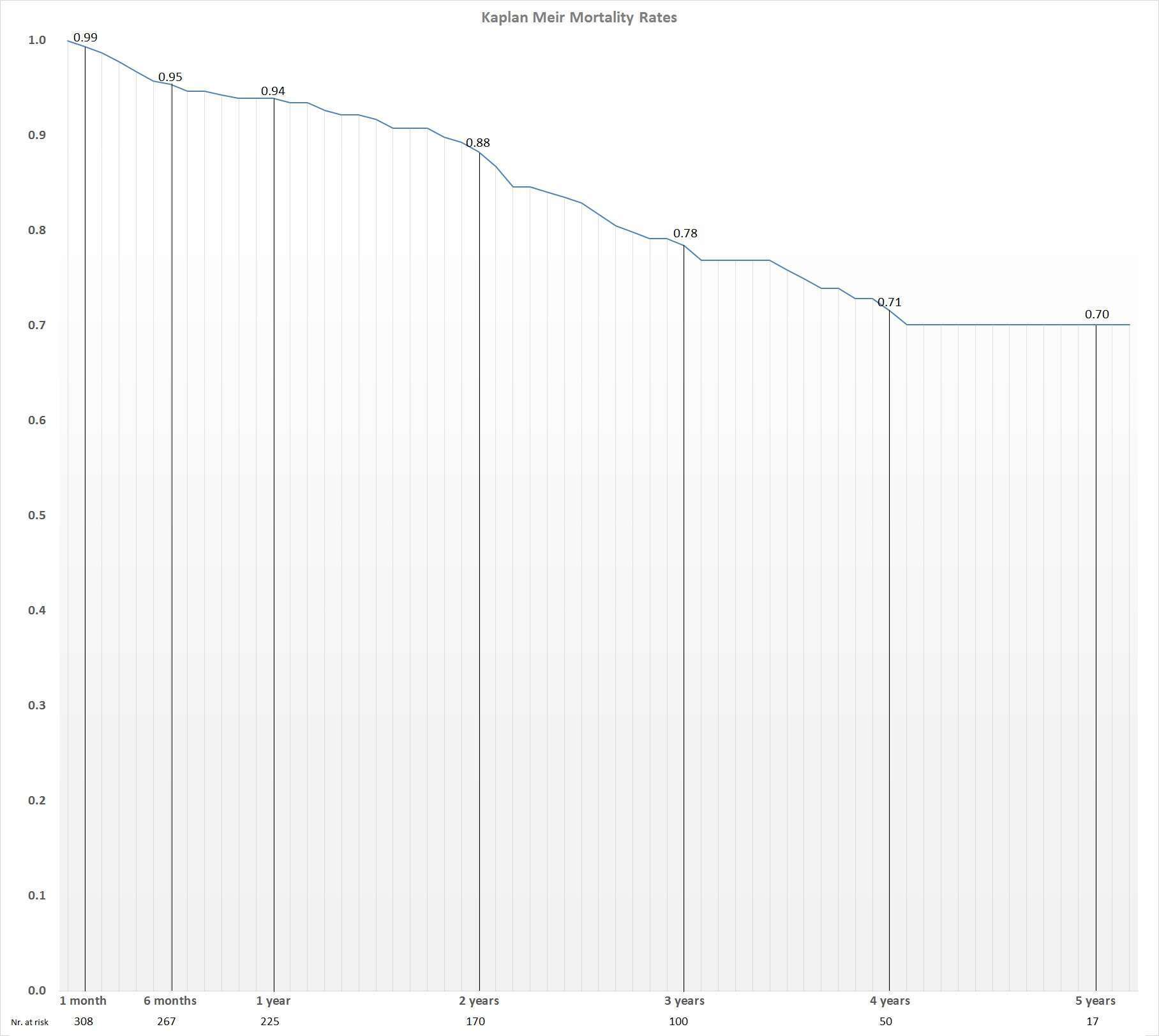
Results This patient group was characterized by advanced age (mean 82±6.4), high mean logistic EuroScore = 19.1±12 and STS score = 8.1±5. The Medtronic-CoreValve®, the Edwards-Sapien®, the Corevalve Evolut R® and the Lotus Valve Systemtm device, were utilized in 230 (70%), 94 (29%), 2 (0.6%) and 3 (0.9%) of patients; respectively. The trans-femoral, trans-axillar, trans-apical, trans-aortic and retroperitoneal route were used in 278, 17, 32, 1 and 1 of the cases; respectively. Procedural success was 96%. However, according to the VARC 2 definitions, device success was achieved in only 92.7% of cases since there was a need for a second valve implant in 11 (3.3%) patients. One month, 1 year, 2 and 3 years and 4 years survival rates were 99%, 95%, 94%, 88% and 78%; respectively. There were 3 (0.9%) patients who needed urgent cardiac surgery. Any vascular complications occurred in 71 (21.5%). Peri-procedural\in-hospital stroke was diagnosed in 14 (4.2%) patients. Permanent pacemaker was required in 53 (16.1%) patients: (49 cases in CoreValve and 4 in Sapien valves). Paravalvular leak (≥moderate) was noted in 32 (10%) patients. The median length of hospital stay was 5±4.3 days. After the procedure, mean valve gradients decreased from 53.6±14 to 7.2±7 (p<0.001). Symptomatic improvement was evident during follow up, having 98% of patients in NYHA-FC I or II.
Conclusions Transcatheter aortic valve implantation is associated with excellent outcomes in elderly patients with severe symptomatic aortic stenosis. This report also demonstrates the long durability of the implanted devices.