
A Fast Assay to Determine Infliximab Trough Level using Fiber-Optic Surface Plasmon Resonance Sensor
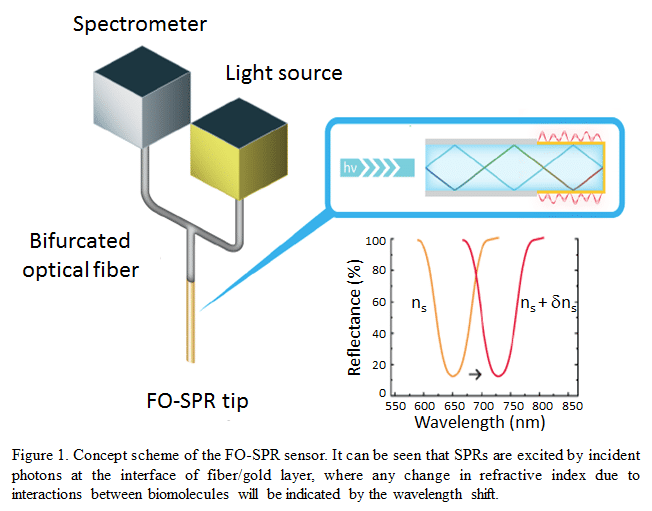
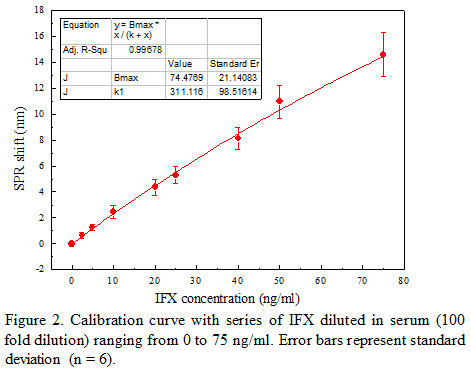
Therapeutic monoclonal antibodies, such as Infliximab (IFX) are highly effective for treating inflammatory bowel disease patients. It is recommended to monitor the trough level of IFX for improving therapeutic outcome of the bimonthly treatment [1]. The conventionally used ELISA assay is time-consuming and not optimal for single patient testing. Here, we present a fiber-optic surface plasmon resonance (FO-SPR) sensor [2] as a potential point-of-care diagnostic tool (Figure 1). On this platform, surface plasmon waves are generated at the interface of an optical fiber/gold layer (50 nm). On top of this gold layer through a self-assembly monolayer (SAM), an in-house developed IFX-specific monoclonal antibody is immobilized to establish a sandwich immunoassay. Analysis was performed on IFX spiked serum in 100 fold diluted serum obtained from healthy volunteers. The signal was specifically amplified by a second IFX-specific detection antibody conjugated to 20 nm gold nanoparticles. An IFX calibration curve was made, ranging from 0 to 75 ng/ml, using a single fiber with surface regeneration in between measurements. The limit of detection from 6 calibration curves (Figure 2) was 2.17 ng/ml, corresponding to 0.22 μg/ml in the whole serum. This bio-assay was further validated with 5 patient samples and corresponding IFX concentrations were determined based on the FO-SPR calibration curves. This work demonstrates the potential of the FO-SPR platform to be used at the point-of-care for improving the therapeutic outcome of patients treated with IFX.
[1] S. Vermeire, A. Gils. Frontline Gastroenterol. Jan 2013; 4(1): 41–43
[2] J. Pollet et al., Talanta 2011; 83: 1436-1441
Powered by Eventact EMS