
Improvement of Plasmon-Induced Water splitting by a Co-Catalyst for Hydrogen Evolution
Noble metal nanoparticles deposited on a semiconductor have the potential to promote light-energy conversion due to the light harvesting effect based on localized surface plasmon resonances because the metal-semiconductor interface separates the photogenerated electrons and holes. We reported on a plasmon-induced water splitting system using both sides of the same strontium titanate (SrTiO3) substrate whereby irradiation with visible light to gold nanoparticles deposited on the substrate separately generate hydrogen (H2) and oxygen (O2) in a stoichiometric ratio. In this study, improvement of the plasmon-induced water splitting system by metal or metal oxide as a co-catalyst for the H2 evolution is demonstrated.
We have prepared a gold nanoislands loaded SrTiO3 substrate on which platinum (Pt) board was stick via ohmic contact as reported previously [1]. Subsequently, two kinds of metal Ru and Rh or metal oxide (RuOx and PhOx) with a thickness of 2 nm was deposited on a Pt board. The water splitting was performed by irradiating visible light (550 – 650 nm) onto gold nanoparticles.
Figure 1 depicts H2 and O2 evolution rates using Pt board decorated with co-catalyst. Chemical bias (0.7 V) was applied by the pH regulations between H2 and O2 evolution sides. We confirmed similarly stoichiometric evolution of H2 and O2 as reported previously [1]. Importantly, the H2 evolution rate has been enhanced by all co-catalysts especially by Rh. They are supposed to facilitate the electron transfer and its reaction with the protons in the acid solution environment. The primary role of noble-metal or metal oxide thin film on the Pt board is to offer active sites for the reduction of proton.
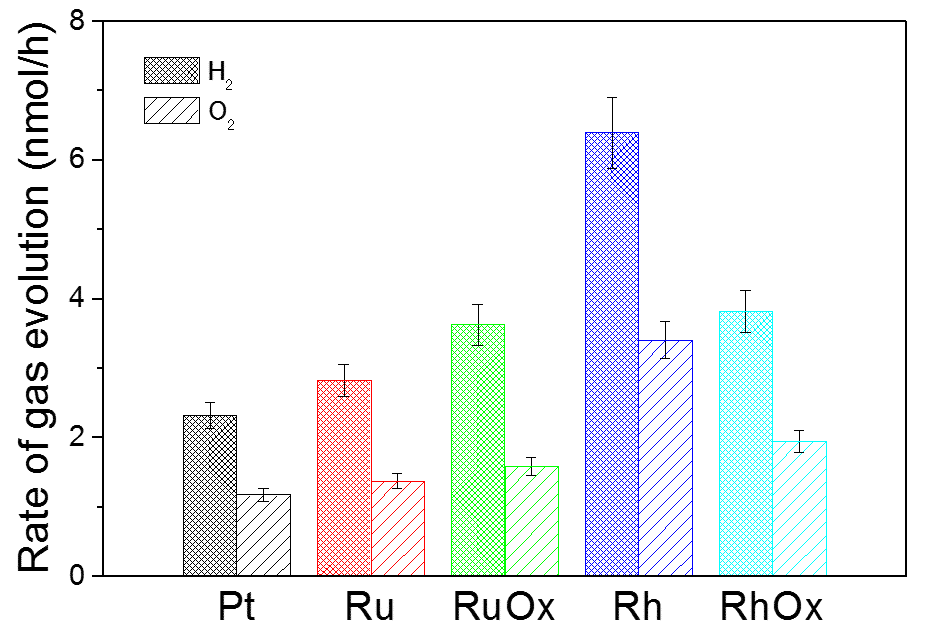
Fig. 1. H2 and O2 evolution rate by the water splitting system using Pt board decorated with various kinds of co-catalyst as the hydrogen evolution site.
[1] H. Misawa et al. Angew. Chem. Int. Ed. 53, 10350 (2014).
misawa@es.hokudai.ac.jp
Powered by Eventact EMS