
UV Fluorescence Lifetime Modification by Al and Mg Nanoapertures
Exploring new materials that can sustain SPPs in the Ultraviolet spectral range has generated a surge of interest recently [1]. One implication is improving the detection limit of biomolecules whose native fluorescence reside in the UV range [2]. Fluorescence lifetime studies serve as the first step towards quantitative fluorescence analysis, and nanoapertures are one of the simplest nanostructures with which to study freely diffusing single molecules. Our group has reported fluorescence lifetime reduction of ∼3.5× for the high quantum yield (QY) UV laser dye p-terphenyl by Al nanoapertures with diameter 60nm [3]. We further showed that lifetime reduction depends upon the native QY of the emitter, with greater reduction occurring for emitter with higher QY, and the amount of undercut beneath the aperture during FIB milling. Here we report investigations of smaller nanoapertures down to 40nm in diameter and compare the properties of Al and Mg.
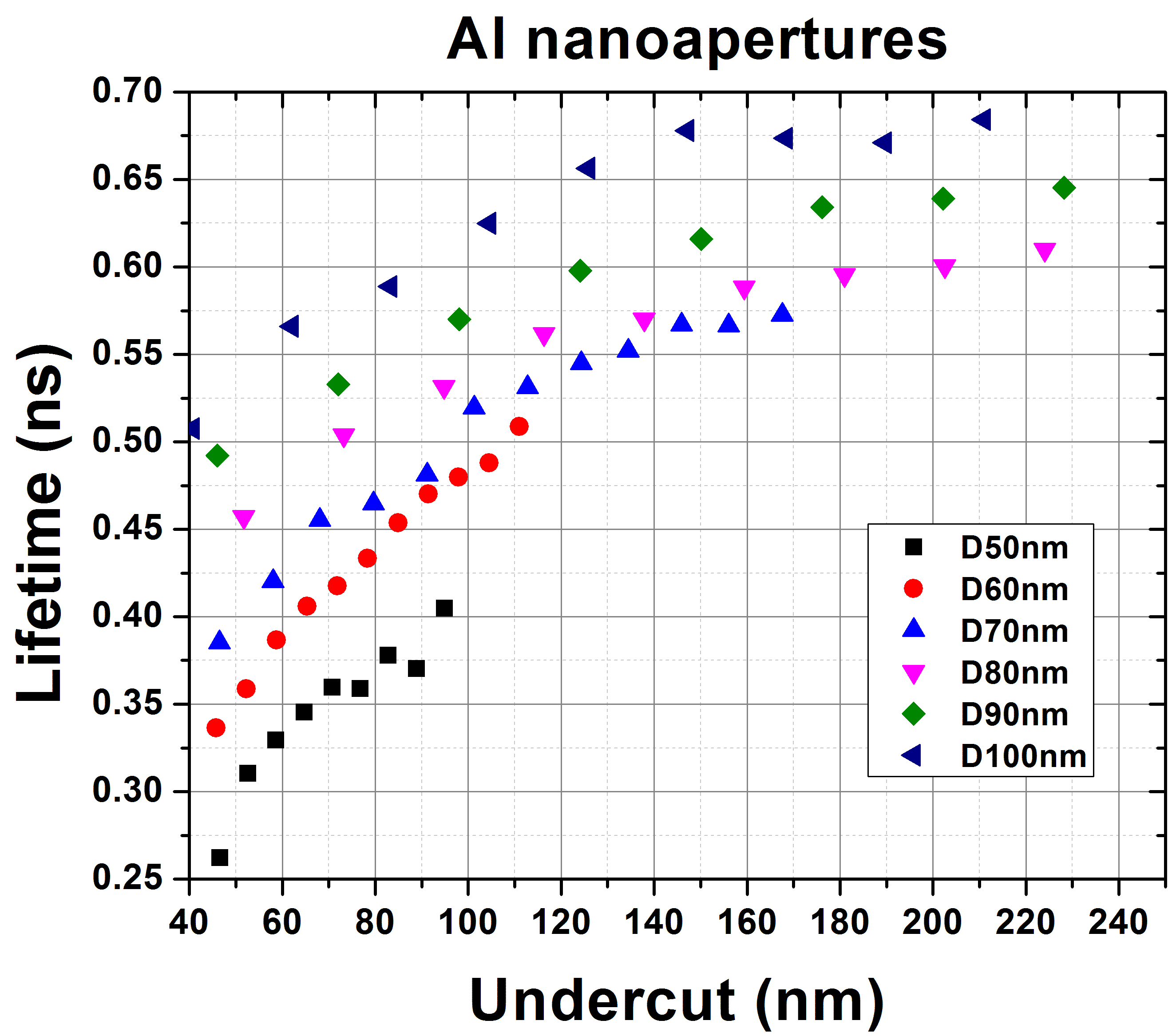
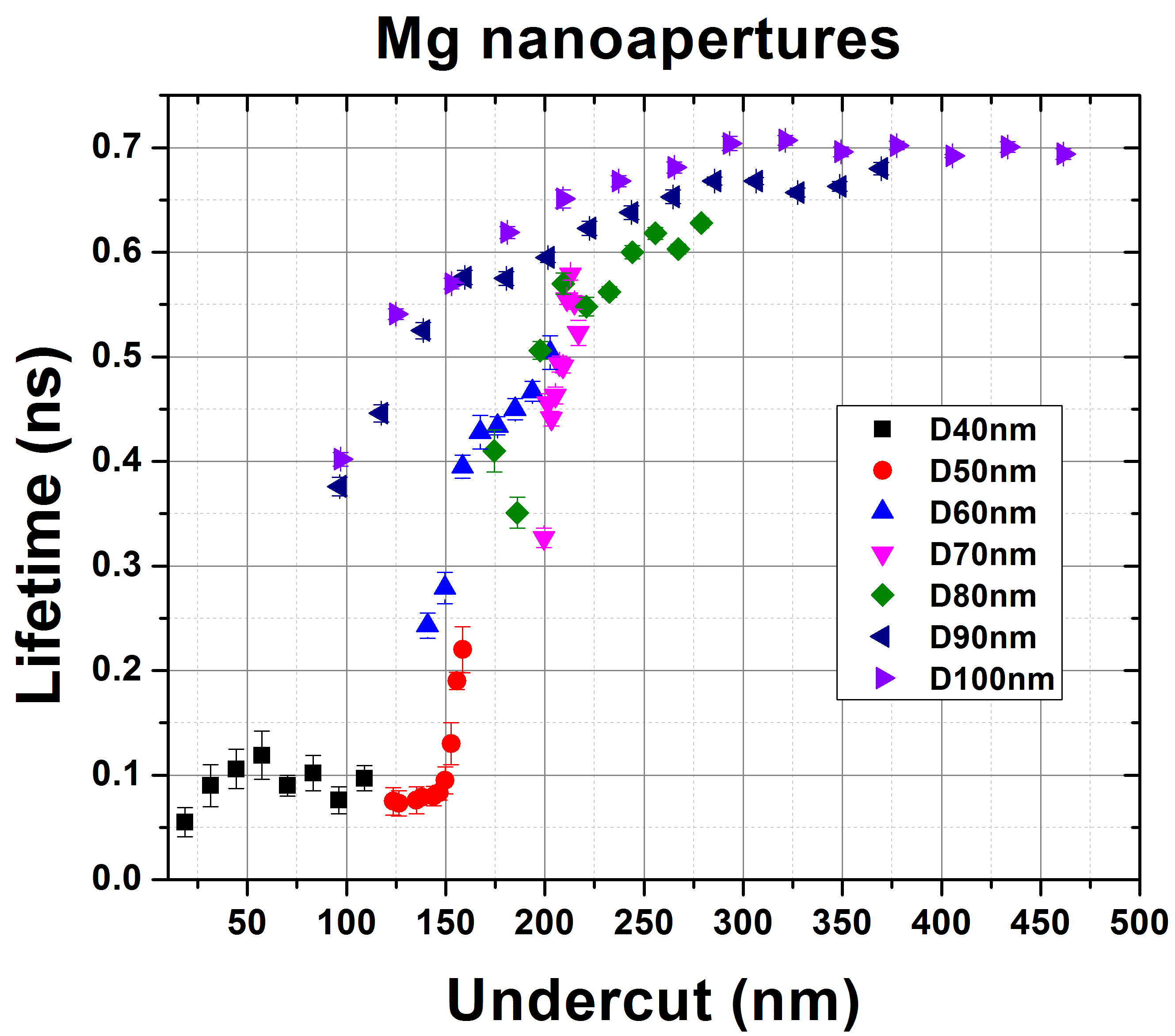
We observed up to ~13× lifetime reduction by Mg nanoapertures with smaller sizes down to 40nm, which is nearly 3× greater than for Al. For larger aperture sizes (60-100nm), Al and Mg nanoapertures exhibit similar lifetime reduction factors. These results are consistent with our FDTD simulations and suggest that Mg nanostructures are promising for modifying the lifetime of UV fluorescent molecules.
Figures: Measured lifetime reduction versus undercut for p-terphenyl inside Al and Mg nanoapertures with different aperture sizes. p-Terphenyl concentration is 100 uM in 1-octanol. Different undercuts into substrate are introduced by varied milling doses.
Reference
[1] M. McMahon, G. C. Schatz, and S. K. Gray, Physical Chemistry Chemical Physics : PCCP, vol. 15, no. 15, pp. 5415-23, 2013
[2] R. Lakowicz, B. Shen, Z. Gryczynski, S. D`Auria, and I. Gryczynski, Biochemical and Biophysical Research Communications, vol. 286, no. 5, pp. 875-9, 2001.
[3] Jiao, E. M. Peterson, J. M. Harris, and S. Blair, ACS Photonics, vol. 1, no. 12, pp. 1270-1277, 2014.
Blair@ece.utah.edu
Powered by Eventact EMS