
Clinical Relevance, Pathological Details and Mechanistic Functions of Desmoplastic 3D-Adhesion Structures
2Computer Science, University of North Carolina
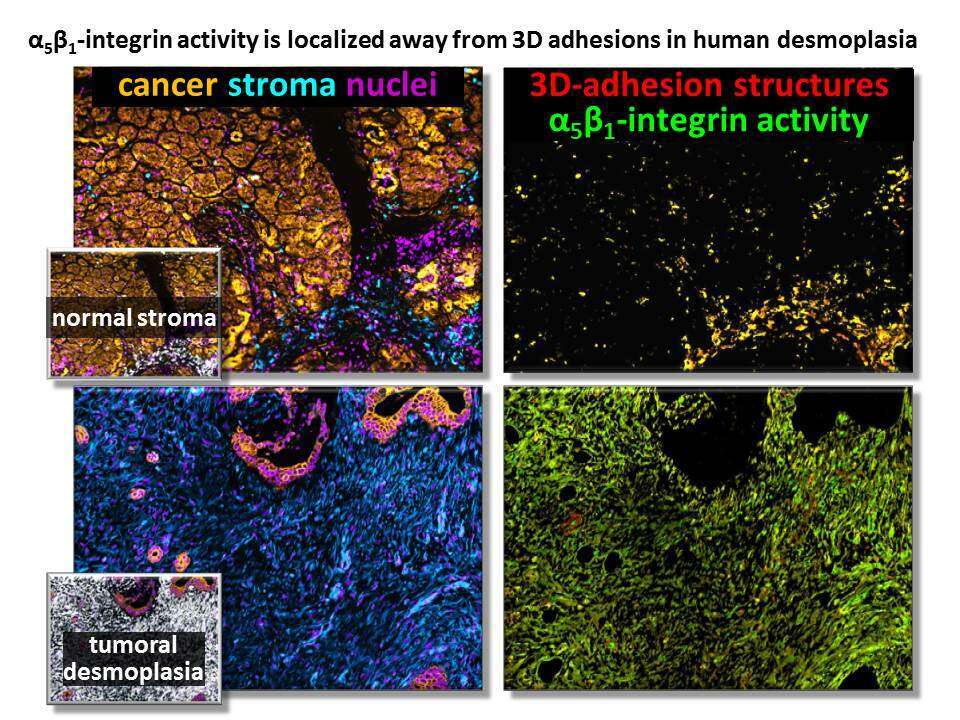
We have demonstrated that desmoplastic extracellular matrices (ECMs) effectively induce an active myofibroblastic phenotype upon naive fibroblasts. Here, we aim to reprogram the desmoplastic microenvironment back to tumor suppressive and query its clinical relevance. Using syngeneic human fibroblasts harvested from pancreatic or renal cancer patient-matched normal and tumor surgical samples, we prompted cells to produce a human mimetic 3D stroma system and attempted reprograming. Approaches included the use of specific inhibitor and activator drugs as well as genetic manipulations. Pathophysiological validations were conducted via simultaneous multi-channel immunofluorescence labeling of the original surgical tissue samples distinguishing tumoral from stromal compartments and questioning the localization and activity levels of α5β1-integrin with regards to desmoplastic 3D-adhesion structures. To establish clinical relevance, the same approach was combined with a novel computational code applied as a batch analysis on human renal and pancreatic tissue cohorts.
We show that, while TGFβ is needed for desmoplastic fibrillogenesis, it is dispensable for desmoplastic ECM-induced myofibroblastic activation. Data point to a mechanism whereby αvβ5-integrin triggers the redistribution of FAK-independent α5β1-integrin activity away from desmoplastic 3D-adhesion structures. This in turn prevents α5β1-integrin from inhibiting the desmoplastic ECM-induced phenotype. Results from the pathological samples suggest that levels of stromal α5β1-integrin activity localized away from desmoplastic 3D-adhesions could foretell recurrence.
We posit that this desmoplastic mechanism not only comprises a clinically important occurrence and novel outcome-predictive and desmoplastic index bio-marker, but it also constitutes a possible new therapeutic aimed at normalizing-desmoplasia. Results from this study consolidate some current controversies regarding the tumorigenic value of desmoplasia.
Funding: Pennsylvania’s Commonwealth, Bucks County Chapter Board of Associates, PanCan Greenberg Family Fund, Keystone Program in Personalized Kidney Cancer, NCI/NIH and DOD.
Powered by Eventact EMS