
THE EFFECT OF SALTS ON THE NANOSTRUCTURE OF SLES AQUEOUS SOLUTIONS OBSERVED BY Cryo-TEM
2Institute of Physical and Theoretical Chemistry, University of Regensburg, Regensburg, Germany
Sodium lauryl ether sulfate (SLES) is an amphiphilic molecule, widely used as an anionic surfactant in soaps, personal care, and cosmetic products. SLES in aqueous solutions, with the addition of minor components, self-assembles into different nano-aggregates, such as nanometric-scale spherical micelles, threadlike micelles, and branched networks (Figure 1), depending on the concentration of SLES or the additive. The nanostructure of SLES is affected by interactions with additives, like salts or fragrance molecules, which are typically present in SLES-based industrial formulations. Direct imaging of SLES with the absence and presence of additives can explain the nanostructural modifications affecting macroscopic properties and behavior. This could serve as a basis for optimized formulations of SLES-based products.
We use cryogenic transmission electron microscopy (cryo-TEM) to study the effect of different salts on the nanostructure of SLES aqueous solutions, at different salt-to-surfactant molar ratios (marked X). We conduct rheological measurements to predict nanostructural changes, as the viscosity of the system is strongly affected by the self-aggregated nanostructure of the system. Here we show the correlation between the rheological properties and the nanostructural changes of the system at varying salt concentrations. We also demonstrate how specimen preparation, particularly the blotting method and specimen relaxation prior to vitrification, affect the imaged nanostructures through artifact formation.
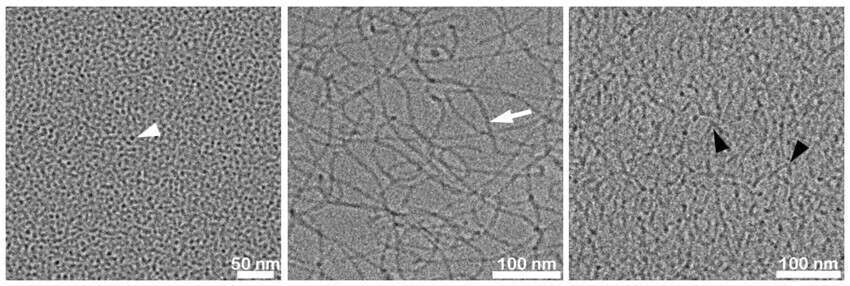
Figure 1. Cryo-TEM micrographs demonstrating the nanostructural transitions of 5 wt.% SLES aqueous solutions with different NaCl ratios. (A) SLES without salt, showing small spheroidal micelles (white arrowhead); (B) X=10, SLES with NaCl at the zero-shear viscosity peak, showing sparse networks of elongated threadlike micelles (white arrow); (C) X=12, dense networks of branched threadlike micelles (branching points marked with black arrowheads).